Catalytic investigation of Pd(II) complexes over Heck-Mizoroki reaction: Tailored synthesis, characterization and density functional theory
Main Article Content
Abstract
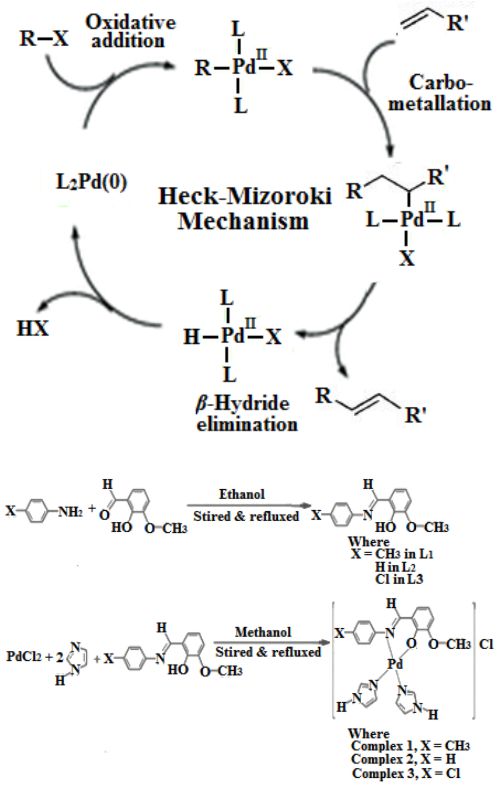
Tailored reaction of Schiff base ligands with palladium(II) chloride and imidazole afford three complexes of formula [Pd(II)(L)(imdz)2]Cl; where L = 2-((E)-(p-lylimino)methyl)-6-methoxyphenol (complex 1); 2-methoxy-6-((E)-(phenylimine)methyl)phenol (complex 2); and 2-((E)-(4-chlorophenylimino)methyl)-6-methoxyphenol (complex 3). Compounds were characterized with elemental analysis, molar conductance, electronic spectroscopy, ESI-MS, FT-IR, TGA, 1H-NMR and 13C-NMR. Molecular structure and different quantum chemical parameters were calculated using the B3LYP basis set of density functional theory with the standard 6-311+G (d, 2p) level. The catalytic potential of 1-3 was examined over Heck-Mizoroki reaction and found in order of 1 > 2 > 3.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
K. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 44 (2005) 4442 (https://doi.org/10.1002/anie.200500368)
C. Jia, T. Kitamura, Y. Fujiwara, Acc. Chem. Res. 34 (2001) 633 (https://dx.doi.org/10.1021/ar000209h)
A. Balanta, C. Godard, C. Claver, Chem. Soc. Rev. 40 (2011) 4973 (https://dx.doi.org/10.1039/c1cs15195a)
A. R. Hajipour, F. Rafiee, J. Organomet. Chem. 696 (2011) 2669 (https://dx.doi.org/10.1016/j.jorganchem.2011.03.023)
A. Dewan, U. Bora, G. Borah, Tet. Lett. 55 (2014) 1689 (https://dx.doi.org/10.1016/j.tetlet.2014.01.041)
F. Bakkali, S. Averbeck, D. Averbeck, M. Idaomar, Food Chem. Toxicol. 46 (2008) 446 (https://dx.doi.org/10.1016/j.fct.2007.09.106)
M. Esmaeilpour, J. Javidi, J. Chin. Chem. Soc. 62 (2015) 614 (http://dx.doi.org/10.1002/jccs.201500013)
R. F. Heck, J. Am. Chem. Soc. 90 (1968) 5518 (https://doi.org/10.1021/ja01022a034)
J. P. Genet, M. Savignac, J. Organomet. Chem. 576 (1999) 305 (https://doi.org/10.1021/ja01022a034)
M. Sankarganesh, N. Revathi, J. D. Raja, K. Sakthikumar, G. G. V. Kumar, J. Rajesh, M. Rajalakshmi, L. Mitu, J. Serb. Chem. Soc. 84 (2019) 291 (https://dx.doi.org/10.2298/JSC180609080)
H. O. Oloyede, J. A. O. Woods, H. Gorls, W. Plass, A. O. Eseola, J. Mol. Struct. 1199 (2020) 1 (https://dx.doi.org/10.1016/j.molstruc.2019.127030)
H. A. Doung, M. Cross, J. Org. Lett. 6 (2004) 4679 (https://dx.doi.org/10.1021/ol048211m)
P. J. Knowles, A. Whiting, Org. Biomol. Chem. 5 (2007) 31 (https://dx.doi.org/10.1039/b611547k)
C. S. Letizia, J. Cocchiara, J. Lalko, A. M. Api, Food Chem. Toxicol. 41 (2003) 943 (https://dx.doi.org/10.1016/S0278-6915(03)00015-2)
G. H. Jeffery, J. Bassett, J. Mendham, R. C. Denney, Vogels Textbook of Quantitative Inorganic Analysis, 5th ed., John Wiley & Sons, Inc. New York, 1989
Y. Y. Yu, H. D. Xian, J. F. Liu, G. L. Zhao, Molecules 14 (2009) 1747 (https://dx.doi.org/10.3390/molecules14051747)
M. Amirnasar, A. H. Mahmoudkhani, A. Gorji, S. Dehghanpour, H. R. Bijanzadeh, Polyhedron 21 (2002) 2733 (https://dx.doi.org/10.1016/S0277-5387(02)01277-9)
N. Raman, Y. P. Raja, A. Kulandaisamy, Proc. Indian Acad. Sci. (Chem. Sci.) 113 (2001) 183
G. Y. Yeap, S. T. Ha, S. N. Ishizawa, K. L. Boey, W. A. K. Mahmood, J. Mol. Struct. 658 (2003) 87 (https://dx.doi.org/10.1016/S0022-2860 (03)00453-8)
Gaussian Inc., Wallingford, CT, 2009
M. Dehestani, L. Zeidabadinejad, J. Serb. Chem. Soc. 80 (2015) 1008 (https://dx.doi.org/10.2298/JSC150224027Z)
D. A. Vicic, G. D. Jones, Experimental Methods and Techniques: Basic Techniques, Elsevier Ltd., University of Arkansas, Fayetteville, AR, 2007
W. J. Geary, J. Coord. Chem. Rev. 7 (1971) 81 (https://dx.doi.org/10.1016/S0010-8545(00)80009-0)
E. G. Bakirdere, M. F. Fellah, E. Canpolat, M. Kaya, S. Gur, J. Serb. Chem. Soc. 81 (2016) 520 (https://doi.org/10.2298/JSC151030008B)
M. Shabbir, Z. Akhter, I. Ahmad, S. Ahmed, M. Shafiq, B. Mirza, V. Mckee, K. S. Munawar, A. R. Ashraf, J. Mol. Struct. 1118 (2016) 250 (https://dx.doi.org/10.1016/j.molstruc.2016.04.003)
A. A. Soliman, I. O. Alajrawy, A. F. Attabi, M. R. Shaaban, W. Linert, Spectrochim. Acta, A 152 (2016) 358 (https://dx.doi.org/10.1016/j.saa.2015.07.076)
Z. Leka, S. Grujic, Z. Tesic, S. Lukic, S. Skuban, S. Trifunovic, J. Serb. Chem. Soc. 69 (2004) 137 (https://doi.org/10.2298/JSC0402137L)
C. V. Barra, F. V. Rocha, A. V. G. Netto, R. C. G. Frem, A. E. Mauro, I. Z. Carlos, S. R. Ananias, M. B. Quilles, J. Therm. Anal. Calorim. 106 (2011) 489 (https://dx.doi.org/10.1007/s10973-011-1393-0)
S. A. Al-Jibori, M. M. Barbooti, M. H. S. Al-Jibori, B. K. Aziz, J. Mater. Environ. Sci. 8 (2017) 1365
V. G. Netto, A. M. Santana, A. E. Mauro, Regina C. G. Frem, J. Therm. Anal. Calorim 79 (2005) 339 (https://doi.org/10.1007/s10973-005-0061-7)
N. Yıldırım, N. Demir, G. Alpaslan, B. Boyacioglu, M. Yıldız, H. Unver, J. Serb. Chem. Soc. 83 (2018) 707 (https://dx.doi.org/10.2298/JSC171001009Y)
T. A. Mohamed, I. A. Shaaban, R. S. Farag, W. M. Zoghaib, M. S. Afifi, Spectrochim. Acta, A 135 (2015) 417 (https://dx.doi.org/10.1016/j.saa.2014.07.018)
J. M. Collinson, Wilton-Ely, J. Cat. Commun. 87 (2016) 78 (https://dx.doi.org/10.1016/j.catcom.2016.09.006)
S. Layek, Anuradha. B. Agrahari, D. D. Pathak, J. Organomet. Chem. 846 (2017) 105 (https://dx.doi.org/10.1016/j.jorganchem.2017.05.049)
R. N. Prabhu, R. Ramesh, Tetrahedron Lett. 53 (2012) 5961 (https://dx.doi.org/10.1016/j.tetlet.2012.08.120)
C. S. Consorti, G. Ebeling, F. R. Flores, F. Rominger, J. Adv. Synth. Catal. 346 (2004) 617 (https://dx.doi.org/10.1002/adsc.200303228).