The spectral study of azo dye and cationic surfactant interaction in ethanol–water mixture Scientific paper
Main Article Content
Abstract
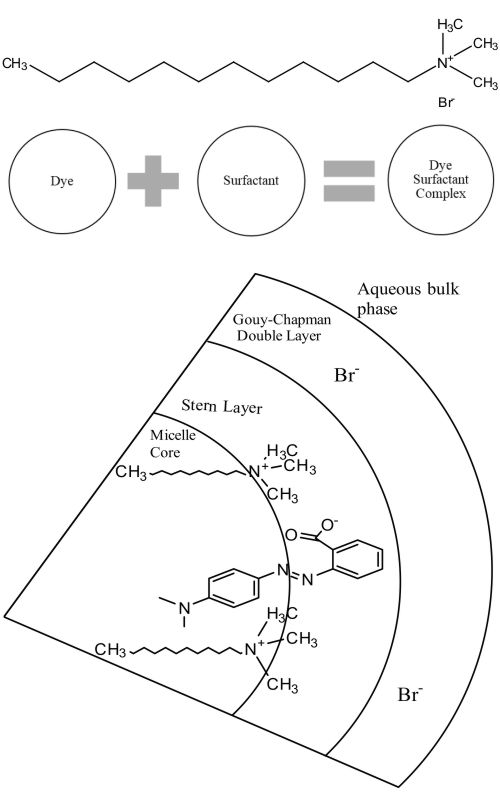
The interaction of the azo dye methyl red (MR) with dodecyl trimethyl ammonium bromide (DTAB) has been studied by the spectrometric methods through the azo-hydrazone tautomeric behaviour of MR for a series of the ethanol–water system (0.1, 0.2, 0.3 and 0.4 volume fractions of ethanol) at room temperature. The critical micelle concentration was determined using the conductometric technique with the increased ethanol volume, influenced by the solvent polarity and the architectural flexibility of methyl red. The azo form of methyl red brings the electrostatic interaction with the cationic surfactant through the adsorption phenomenon. The binding parameters were calculated with the aid of a modified Benesi-Hildebrand equation.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
G. Kyzas, E. Peleka, E. Deliyanni, Materials 6 (2013) 184 (https://doi.org/10.3390/ma6010184)
A. Bhattarai, A. K. Yadav, S. K. Sah, A. Deo, J. Mol. Liq. 242 (2017) 831 (https://doi.org/10.1016/j.molliq.2017.07.085)
A. Pal, A. Garain, D. Chowdhury, M. H. Mondal, B. Saha, Tenside Surfact. Det. 57 (2020) 401 (https://doi.org/10.3139/113.110700)
S. Tunç, O. Duman, B. Kancı, Dyes Pigments 94 (2012) 233 (https://doi.org/10.1016/j.dyepig.2012.01.016)
K. M. Sachin, S. A. Karpe, M. Singh, A. Bhattarai, Roy. Soc. Open Sci. 6 (2019) 181979 (https://doi.org/10.1098/rsos.181979)
S. Fazeli, B. Sohrabi, A. R. Tehrani-Bagha, Dyes Pigments 95 (2012) 768 (https://doi.org/10.1016/j.dyepig.2012.03.022)
H. Akbaş, Ç. Kartal, Spectrochim. Acta, A 61 (2005) 961 (https://doi.org/10.1016/j.saa.2004.05.025)
S. Malik, A. Ghosh, P. Sar, M. H. Mondal, K. Mahali, B. Saha, J. Chem. Sci. 129 (2017) 637 (https://doi.org/10.1007/s12039-017-1276-4)
M. F. Nazar, S. S. Shah, M. A. Khosa, J. Surfact. Deterg. 13 (2010) 529 (https://doi.org/10.1007/s11743-009-1177-8)
M. H. Mondal, S. Malik, B. Saha, Tenside Surfact. Det. 54 (2017) 378 (https://doi.org/10.3139/113.110519)
M. Mondal, M. Ali, A. Pal, B. Saha, Tenside Surfact. Det. 56 (2019) 516 (https://doi.org/10.3139/113.110654)
S. Biswas, A. Pal, Talanta 206 (2020) 120238 (https://doi.org/10.1016/j.talanta.2019.120238)
F. Ahmadi, M. A. Daneshmehr, M. Rahimi, Spectrochim. Acta, A 67 (2007) 412 (https://doi.org/10.1016/j.saa.2006.07.033)
S. Sharifi, M. F. Nazar, F. Rakhshanizadeh, S. A. Sangsefedi, A. Azarpour, Opt. Quantum Elec. 52 (2020) (https://doi.org/10.1007/s11082-020-2211-3)
C. Hahn, A. Wokaun, Langmuir 13 (1997) 391 (https://doi.org/10.1021/la9603378)
S. Chanda, K. Ismail, IJC A 48 (2009) 775 (http://nopr.niscair.res.in/handle/123456789/4686)
M. H. Mondal, S. Malik, A. Roy, R. Saha, B. Saha, RSC Adv. 5 (2015) 92707 (https://doi.org/10.1039/c5ra18462b)
R. B. Narayan, R. Goutham, B. Srikanth, K. P. Gopinath, J. Environ. Chem. Eng. 6 (2018) 3640 (https://doi.org/10.1016/j.jece.2016.12.004)
L. Zhang, J. M. Cole, P. G. Waddell, K. S. Low, X. Liu, ACS Sustain. Chem. Eng. 1 (2013) 1440 (https://doi.org/10.1021/sc400183t)
D. N. Christodoulides, I. C. Khoo, G. J. Salamo, G. I. Stegeman, E. W. Van Stryland, Adv. Opt. Photonics 2 (2010) 60 (https://doi.org/10.1364/aop.2.000060)
M. R. Plutino, E. Guido, C. Colleoni, G. Rosace, Sens. Actuators, B 238 (2017) 281 (https://doi.org/10.1016/j.snb.2016.07.050)
K. M. Sachin, S. A. Karpe, M. Singh, A. Bhattarai, Heliyon 5 (2019) e01510 (https://doi.org/10.1016/j.heliyon.2019.e01510)
A. A. Rafati, S. Azizian, M. Chahardoli, J. Mol. Liq. 137 (2008) 80 (https://doi.org/10.1016/j.molliq.2007.03.013)
M. S. Ramadan, N. M. El-mallah, G. M. Nabil, M. Sherif, J. Dispers. Sci. Technol. 40 (2018) 1 ( https://doi.org/10.1080/01932691.2018.1496837)
K. K. Karukstis, J. P. Litz, M. B. Garber, L. M. Angell, G. K. Korir, Spectrochim. Acta, A 75 (2010) 1354 (https://doi.org/10.1016/j.saa.2009.12.087)
M. L. Moyá, A. Rodríguez, M. del Mar Graciani, G. Fernández, J. Colloid Interf. Sci. 316 (2007) 787 (https://doi.org/10.1016/j.jcis.2007.07.035)
M. E. D. Garcia, A. Sanz-Medel, Talanta 33 (1986) 255 (https://doi.org/10.1016/0039-9140(86)80060-1)
S. K. Shah, S. K. Chatterjee, A. Bhattarai, J. Mol. Liq. 222 (2016) 906 (https://doi.org/10.1016/j.molliq.2016.07.098)
K. Edbey, A. El-Hashani, A. Benhmid, K. Ghwel, M. Benamer, Chem. Sci. Int. J. 24 (2018) 1 (https://doi.org/10.9734/csji/2018/44312)
P. Shah, N. Jha, A. Bhattarai, J. Chem-Ny. 2020 (2020) 5292385 (https://doi.org/10.1155/2020/5292385)
A. Rodríguez, M. del M. Graciani, M. L. Moyá, Langmuir 24 (2008) 12785 (https://doi.org/10.1021/la802320s)
S. Brac̆ko, J. Špan, Dyes Pigments 50 (2001) 77 (https://doi.org/10.1016/S0143-7208(01)00025-0).