Fluoride ion conductivity of solid solutions KxPb0.86-xSn1.14F4-x Scientific paper
Main Article Content
Abstract
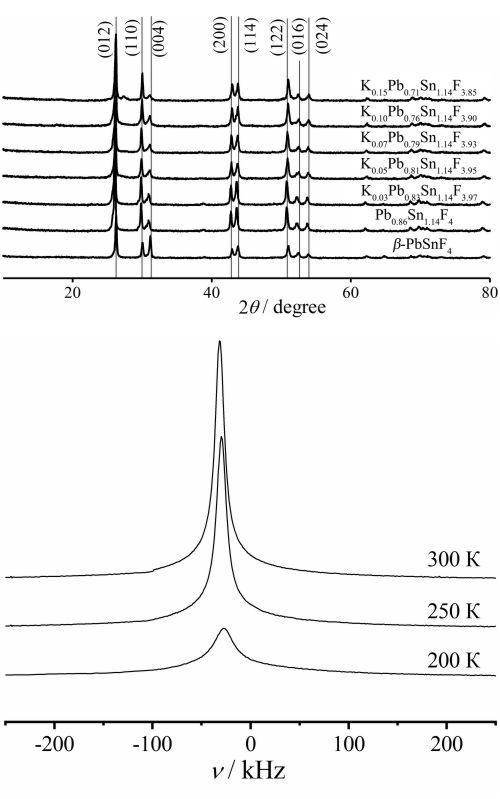
The electrical conductivity of solid solutions with tetragonal syngony formed in 0.86(xKF–(1–x)PbF2)–1.14SnF2 systems has been studied by 19F-NMR and impedance spectroscopy. It was found that the Pb0.86Sn1.14F4 phase is characterized by better values of fluoride-ion conductivity than the β-PbSnF4 compound. It was found that the substitution of Pb2+ by K+ up to х = 0.07 in the structure of Pb0.86Sn1.14F4 contributes to increase in electrical conductivity by an order of magnitude relative to the original Pb0.86Sn1.14F4. The sample of composition K0.03Pb0.83Sn1.14F3.97 has the highest electrical conductivity (σ600 = 0.38 S cm-1, σ330 = 0.01 S cm-1). The fluoride anions in the synthesized samples of KxPb0.86-xSn1.14F4-x solid solutions occupy three structurally nonequivalent positions. It is shown that with increasing temperature, there is a redistribution of fluorine anions between positions in the anion lattice, which results in an increase in the concentration of highly mobile fluoride ions, which determine the electrical conductivity of the samples.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
V. Trnovcová, P. P. Fedorov, I. Furár, Russ. J. Electrochem. 45 (2009) 630 (https://doi.org/10.1134/S1023193509060020)
T. Nakajima, H. Groult, Advanced Fluoride-Based Materials for Energy Conversion, Elsevier, Amsterdam, 2015 (https://doi.org/10.1016/C2013-0-18650-3)
F. Gschwind, G. Rodriguez-Garcia, D. J. S. Sandbeck, A. Gross, M. Weil, M. Fichtner, N. Hörmann, J. Fluorine Chem. 182 (2016) 76 (https://doi.org/10.1016/j.jfluchem.2015.12.002)
J. W. Fergus, Sensors Actuators, B 42 (1997) 119 (https://doi.org/10.1016/S0925-4005(97)00193-7)
X. Na, W. Niu, H. Li, J. Xie, Sensors Actuators, B 87 (2002) 222 (https://doi.org/10.1016/S0925-4005(02)00238-1)
W. Moritz, S. Krause, U. Roth, J. Xie, Anal. Chim. Acta 437 (2001) 183
X. D. Wang, W. Shen, R. W. Cattrall, G. L. Nyberg, J. Lieseganget, Aust. J. Chem. 49 (1996) 897 (https://doi.org/10.1071/CH9960897)
M. Fouskaki, S. Sotiropoulou, M. Kosi, N. A. Chaniotakis, Anal. Chim. Acta 478 (2003) 77 (https://doi.org/10.1016/S0003-2670(02)01481-2)
L. Zhang, M. A. Reddy, M. Fichtner, Solid State Ionics 272 (2015) 39 (https://doi.org/10.1016/j.ssi.2014.12.010)
C. B. Alcock, L. Baozhen, Solid State Ionics 39 (1990) 245 (https://doi.org/10.1016/0167-2738(90)90403-E)
C. Rongeat, M.A. Reddy, R. Witter, M. Fichtner, J. Phys. Chem. 117 (2013) 4943 (https://doi.org/10.1021/jp3117825)
A. Potanin, J. Russ. Chem. Soc. 45 (2001) 58
L. N. Patro, K. Hariharan, Solid State Ionics 239 (2013) 41 (https://doi.org/10.1016/j.ssi.2013.03.009)
N. I. Sorokin, B. P. Sobolev, Crystallogr. Rep. 52 (2007) 842 (https://doi.org/10.1134/S1063774507050148)
N.I. Sorokin, Inorg. Mater. 40 (2004) 989 (https://doi.org/10.1023/B:INMA.0000041335.17098.d1)
N. I. Sorokin, P. P. Fedorov, B. P. Sobolev, Inorg. Mater. 33 (1997) 1
A. M. Vakulenko, E. A. Ukshe, Sov. Electrochem. 28 (1992) 1025.
P. P. Fedorov, V. K. Goncharuk, I. G. Maslennikova, I. A. Telin, T. Y. Glazunova, Russ. J. Inorg. Chem. 61 (2016) 239 (https://doi.org/10.1134/S0036023616020078)
R. Kanno, S. Nakamura, Y. Kawamoto, Solid State Ionics 51 (1992) 53 (https://doi.org/10.1016/0167-2738(92)90343-N)
Yu. V. Pohorenko, R. M. Pshenychnyi, A. O. Omelchuk, V. V. Trachevskyi, Solid State Ionics 338 (2019) 80 (https://doi.org/10.1016/j.ssi.2019.05.001)
Y. V. Pogorenko, R. M. Pshenichnyi, V. I. Lutsyk, A. O. Omel'chuk, IOP Conf. Ser.: Mater. Sci. Eng. 175 (2017) 012039 (https://doi.org/10.1088/1757-899X/175/1/012039)
Yu. V. Pohorenko, A. A. Nahorny, R. M. Pshenychnyi, A. O. Omelchuk, Voprosy Khim. Khim. Tekhnol. 5 (2019) 112 (http://dx.doi.org/10.32434/0321-4095-2019-126-5-112-117)
V. M. Goldschmidt, Naturwissenschaften 14 (1926) 477 (https://doi.org/10.1007/BF01507527)
Y. Ito, T. Mukoyama, H. Funatomi, S. Yoshikado, T. Takana, Solid State Ionics 67 (1994) 301 (https://doi.org/10.1016/0167-2738(94)90021-3)
A. K. Jonscher, Nature 267 (1977) 673 (https://doi.org/10.1038/267673a0)
J. T. S. Irvine, D. C. Sinclair, A. R. West, Adv. Mater. 2 (1990) 132 (https://doi.org/10.1002/adma.19900020304)
M. El Omari, J. Senegas, J. M. Réau, Solid State Ionics 107 (1998) 281 (https://doi.org/10.1016/S0167-2738(97)00535-3)
M. M. Ahmad, K. Yamada, T. Okuda, J. Phys. Condens. Matter. 14 (2002) 7233 (https://iopscience.iop.org/article/10.1088/0953-8984/14/30/312)
E. Barsoukov, J. R. Macdonald, Impedance spectroscopy emphasizing solid materials and systems, John Wiley and Sons, Inc., Hoboken, NJ, 2005. (ISBN: 0-471-64749-7)
S. Vilminot, G. Perez, W. Granier, L. Cot, Solid State Ionics 2 (1981) 91 (https://doi.org/10.1016/0167-2738(81)90004-7)
M. G. Izosimova, A. I. Livshits, V. M. Buznik, P. P. Fedorov, E. A. Krivandina, B. P. Sobolev, Sov. Phys.-Sol. State 28 (1986) 2644.
V. Ya. Kavun, A. I. Ryabov, I. A. Telin, A. B. Podgorbunskii, S. L. Sinebryukhov, S. V. Gnedenkov, V.K. Goncharuk, J. Struct. Chem. 53 (2012) 290 (https://doi.org/10.1134/S0022476612020126)
S. P. Gabuda, Yu.V. Gagarinsky, S. A. Polishchuk, NMR in inorganic fluorides, Atomizdat, Moscow, 1978
V. Ya. Kavun, N. F. Uvarov, A. B. Slobodyuk, M. M. Polyantsev, A. S. Ulihin, E. B. Merkulov, V. K. Goncharuk, Solid State Ionics 330 (2019) 1 (https://doi.org/10.1016/j.ssi.2018.12.004)
D. P. Almond, A. R. West, Solid State Ionics 9–10 (1983) 277 (https://doi.org/10.1016/0167-2738(83)90247-3)
Sh. Yoshikado, Y. Ito, J. M. Réau, Solid State Ionics 154–155 (2002) 503 (https://doi.org/10.1016/S0167-2738(02)00489-7)
C. Martineau, F. Fayon, C. Legein, J. Y. Buzaré, G. Corbel, Chem. Mater. 22 (2010) 1585 (https://doi.org/10.1021/cm9030182).