Kinetics of the exchange of water absorbed in silica hydrogel with ethanol: Modelling by Brouers and Sotolongo–Costa fractal kinetics Scientific paper
Main Article Content
Abstract
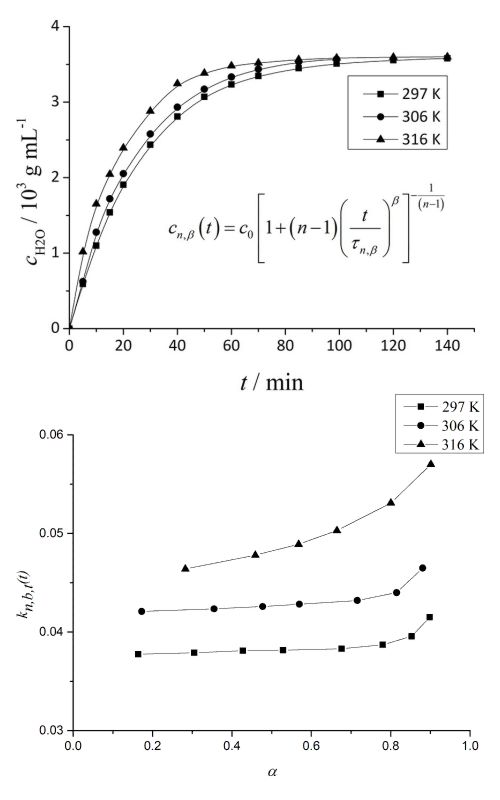
Isothermal kinetics of the exchange of absorbed water in a silica hydrogel (SH) with ethanol was examined. The isothermal kinetic curves of absorbed water exchange with ethanol were measured at the temperatures: T = 297, 306 and 316 K. The rate of the exchange was analysed as a function of time. The possibility of mathematical description of the kinetics of exchange by the Brouers and Sotolongo–Costas (BS) fractals kinetics model was examined. Parameter values (n, τ, β) of the model and their changes with temperature were calculated. By applying the method of Ozao, it was determined that the rate limiting step of the process of exchange was the rate of exchange of the absorbed water with ethanol. Values of the fractal dimension of the SH–ethanol interphase were calculated. The dependences of the effective time-dependent rate coefficient, activation energy and pre-exponential factor on time and degree of exchange were calculated and discussed. The proposed model of the mechanism of the exchange of absorbed water with ethanol was discussed.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
A. C. Pierre, G. M. Pajonk, Chem. Rev. 102 (2002) 4243 (http://dx.doi.org/10.1021/cr0101306)
A. Parvathy Rao, A. Venkateswara Rao, J. Mater. Sci. 45 (2010) 51 (http://dx.doi.org/10.1007/s10853-009-3888-7)
V. G. Parale, K.-Y. Lee, H.-H. Park, J. Korean Ceram. Soc. 54 (2017) 184 (http://dx.doi.org/10.4191/kcers.2017.54.3.12)
E. Moretti, F. Merli, E. Cuce, C. Buratti, Energy Procedia 111 (2017) 472 (http://dx.doi.org/10.1016/j.egypro.2017.03.209)
J. E. Amonette, J. Matyáš, Microporous Mesoporous Mater. 250 (2017) 100 (http://dx.doi.org/10.1016/j.micromeso.2017.04.055)
A. Venkateswara Rao, N. D. Hegde, H. Hirashima, J. Colloid Interface Sci. 305 (2007) 124 (http://dx.doi.org/10.1016/j.jcis.2006.09.025)
S. M. Jones, J. Sol-Gel Sci. Technol. 40 (2006) 351 (http://dx.doi.org/10.1007/s10971-006-7762-7)
C. A. McCarthy, R. J. Ahern, K. J. Devine, A. M. Crean, Mol. Pharm. 15 (2018) 141 (http://dx.doi.org/10.1021/acs.molpharmaceut.7b00778)
C.-T. Wang, C.-L. Wu, I.-C. Chen, Y.-H. Huang, Sensors Actuators, B 107 (2005) 402 (http://dx.doi.org/10.1016/j.snb.2004.10.034)
J. L. Gurav, I.-K. Jung, H.-H. Park, E. S. Kang, D. Y. Nadargi, J. Nanomater. 2010 (2010) 1 (http://dx.doi.org/10.1155/2010/409310)
A. Parvathy Rao, A. Venkateswara Rao, G. M. Pajonk, P. M. Shewale, J. Mater. Sci. 42 (2007) 8418 (http://dx.doi.org/10.1007/s10853-007-1788-2)
J. Šesták, J. Therm. Anal. Calorim. 110 (2012) 5 (http://dx.doi.org/10.1007/s10973-011-2089-1)
S. Gaspard, S. Altenor, N. Passe-Coutrin, A. Ouensanga, F. Brouers, Water Res. 40 (2006) 3467 (http://dx.doi.org/10.1016/j.watres.2006.07.018)
F. Brouers, O. Sotolongo-Costa, Physica, A 368 (2006) 165 (http://dx.doi.org/10.1016/j.physa.2005.12.062)
F. Brouers, J. Mod. Phys. 05 (2014) 1594 (http://dx.doi.org/10.4236/jmp.2014.516160)
R. Ozao, M. Ochiai, J. Ceram. Soc. Japan 101 (1993) 263 (http://dx.doi.org/10.2109/jcersj.101.263)
P. Šimon, O. Zmeškal, J. Šesták, in Thermal analysis of Micro, Nano- and Non-Crys-talline Materials. Hot Topics in Thermal Analysis and Calorimetry, Vol. 9, J. Šesták., P. Šimon, Eds., Springer, Dordrecht, 2012, pp. 247–255 (http://dx.doi.org/10.1007/978-90-481-3150-1_12)
W. Siebrand, T. A. Wildman, Acc. Chem. Res. 19 (1986) 238 (http://dx.doi.org/10.1021/ar00128a002)
A. Plonka, A. Paszkiewicz, J. Chem. Phys. 96 (1992) 1128 (http://dx.doi.org/10.1063/1.462199)
R. Grima, S. Schnell, Biophys. Chem. 124 (2006) 1 (http://dx.doi.org/10.1016/j.bpc.2006.04.019).