Synthesis of new derivatives of alepterolic acid via click chemistry Scientific paper
Main Article Content
Abstract
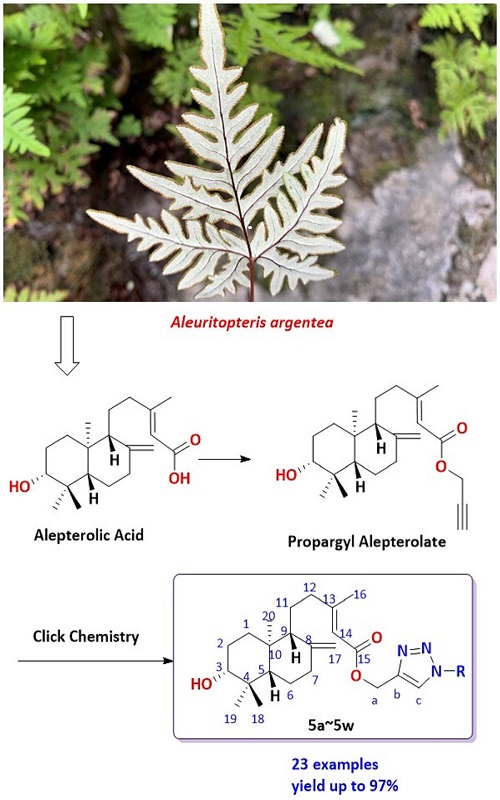
Alepterolic acid is a natural diterpenoid isolated from Aleuritopteris argentea (S. G. Gmél.) Fée, a fern with potential medicinal activity, used in China as a folk medicine to regulate menstruation and prevent cancer. Nevertheless, there are few reports about the structural modification of this natural product. With the wide application of 1,2,3-triazole derivatives in medicines, pesticides, functional materials, the synthesis of 1,2,3-triazoles derivatives has attracted the attention of synthetic chemists. In this article, 23 new derivatives of alepterolic acid combined with 1,2,3-triazole were designed and synthesized by esterification and click chemistry reaction in a fast, conventional and efficient way. All the products were obtained in good yields (72 to 97 %). The structure of these compounds was confirmed by 1H-, 13C-NMR and mass spectral data. The use of the easily available reactants and the common reaction conditions furnish an efficient method for the synthesis of alepterolic acid derivatives. The preparation of these compounds would enable further biological evaluation in the future.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Ageta, K. Awata, Y. Otake, Proc. Symp. Nat. Org. Compd. 6 (1962) 136 (https://doi.org/10.24496/tennenyuki.6.0_136)
E. Wollenweber, P. Rüedi, D. S. Seigler, Z. Naturforsch., C 37 (1982) 1283 (https://doi.org/10.1515/znc-1982-11-1231)
N. D. Idippily, Q. Zheng, C. Gan, A. Quamine, M. M. Ashcraft, B. Zhong, B. Su, Bioorg. Med. Chem. Lett. 27 (2017) 2292 (https://doi.org/10.1016/j.bmcl.2017.04.046)
Z. Xu, S.-j. Zhao, Y. Liu, Eur. J. Med. Chem. 183 (2019) 111700 (https://doi.org/10.1016/j.ejmech.2019.111700)
F. Reck, F. Zhou, M. Girardot, G. Kern, C. J. Eyermann, N. J. Hales, R. R. Ramsay, M. B. Gravestock, J. Med. Chem. 48 (2005) 499 (https://doi.org/10.1021/jm0400810)
R. Raj, P. Singh, P. Singh, J. Gut, P. J. Rosenthal, V. Kumar, Eur. J. Med. Chem. 62 (2013) 590 (https://doi.org/10.1016/j.ejmech.2013.01.032)
C. X. Tan, Y. X. Shi, J. Q. Weng, X. H. Liu, W. G. Zhao, B. J. Li, J. Heterocycl. Chem. 51 (2014) 690 (https://doi.org/10.1002/jhet.1656)
L.-h. Zhou, A. Amer, M. Korn, R. Burda, J. Balzarini, E. De Clercq, E. R. Kern, P. F. Torrence, Antivir. Chem. Chemother. 16 (2005) 375 (https://doi.org/10.1177/095632020501600604)
F. Mir, S. Shafi, M. Zaman, N. P. Kalia, V. S. Rajput, C. Mulakayala, N. Mulakayala, I. A. Khan, M. Alam, Eur. J. Med. Chem. 76 (2014) 274 (https://doi.org/10.1016/j.ejmech.2014.02.017)
L.-y. Ma, L.-p. Pang, B. Wang, M. Zhang, B. Hu, D.-q. Xue, K.-p. Shao, B.-l. Zhang, Y. Liu, E. Zhang, Eur. J. Med. Chem. 86 (2014) 368 (https://doi.org/10.1016/j.ejmech.2014.08.010)
D. Dheer, V. Singh, R. Shankar, Bioorg. Chem. 71 (2017) 30 (https://doi.org/10.1016/j.bioorg.2017.01.010)
K. Bozorov, J. Zhao, H. A. Aisa, Bioorg. Med. Chem. 27 (2019) 3511 (https://doi.org/10.1016/j.bmc.2019.07.005)
Y. W. He, C. Z. Dong, J. Y. Zhao, L. L. Ma, Y. H. Li, H. A. Aisa, Eur. J. Med. Chem. 76 (2014) 245 (https://doi.org10.1016/j.ejmech.2014.02.029)
M. W. Pertino, C. Theoduloz, E. Butassi, S. Zacchino, G. Schmeda-Hirschmann, Molecules 20 (2015) 8666 (https://doi.org/10.3390/molecules20058666)
T. Kasemsuk, N. Saehlim, P. Arsakhant, G. Sittithumcharee, S. Okada, R. Saeeng, Bioorg. Med. Chem. 29 (2021) 115886 (https://doi.org/10.1016/j.bmc.2020.115886)
G. Wei, W. Luan, S. Wang, S. Cui, F. Li, Y. Liu, Y. Liu, M. Cheng, Org. Biomol. Chem. 13 (2015) 1507 (https://doi.org/10.1039/C4OB01605J)
S. Zhang, N. Feng, J. Huang, M. Wang, L. Zhang, J. Yu, X. Dai, J. Cao, G. Huang, Bioorg. Chem. 98 (2020) 103756 (https://doi.org/10.1016/j.bioorg.2020.103756)
J. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 36 (2007) 1249 (https://doi.org/10.1039/B613014N)
C. Wang, L. Lu, H. Na, X. Li, Q. Wang, X. Jiang, X. Xu, F. Yu, T. Zhang, J. Li, Z. Zhang, B. Zheng, G. Liang, L. Cai, S. Jiang, K. Liu, J. Med. Chem. 57 (2014) 7342 (https://doi.org/10.1021/jm500763m)
J.-W. Zhao, J.-W. Guo, M.-J. Huang, Y.-Z. You, Z.-H. Wu, H.-M. Liu, L.-H. Huang, Steroids 150 (2019) 108431 (https://doi.org/10.1016/j.steroids.2019.108431)
P. Shanmugavelan, S. Nagarajan, M. Sathishkumar, A. Ponnuswamy, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 21 (2011) 7273 (https://doi.org/10.1016/j.bmcl.2011.10.048)
X. Meng, X. Xu, T. Gao, B. Chen, Eur. J. Org. Chem. (2010) 5409 (https://doi.org/10.1002/ejoc.201000610).