Synthesis of novel fluorinated 1,5-benzothiazepine derivatives and their biological evaluation as anticancer and antibacterial agents Scientific paper
Main Article Content
Abstract
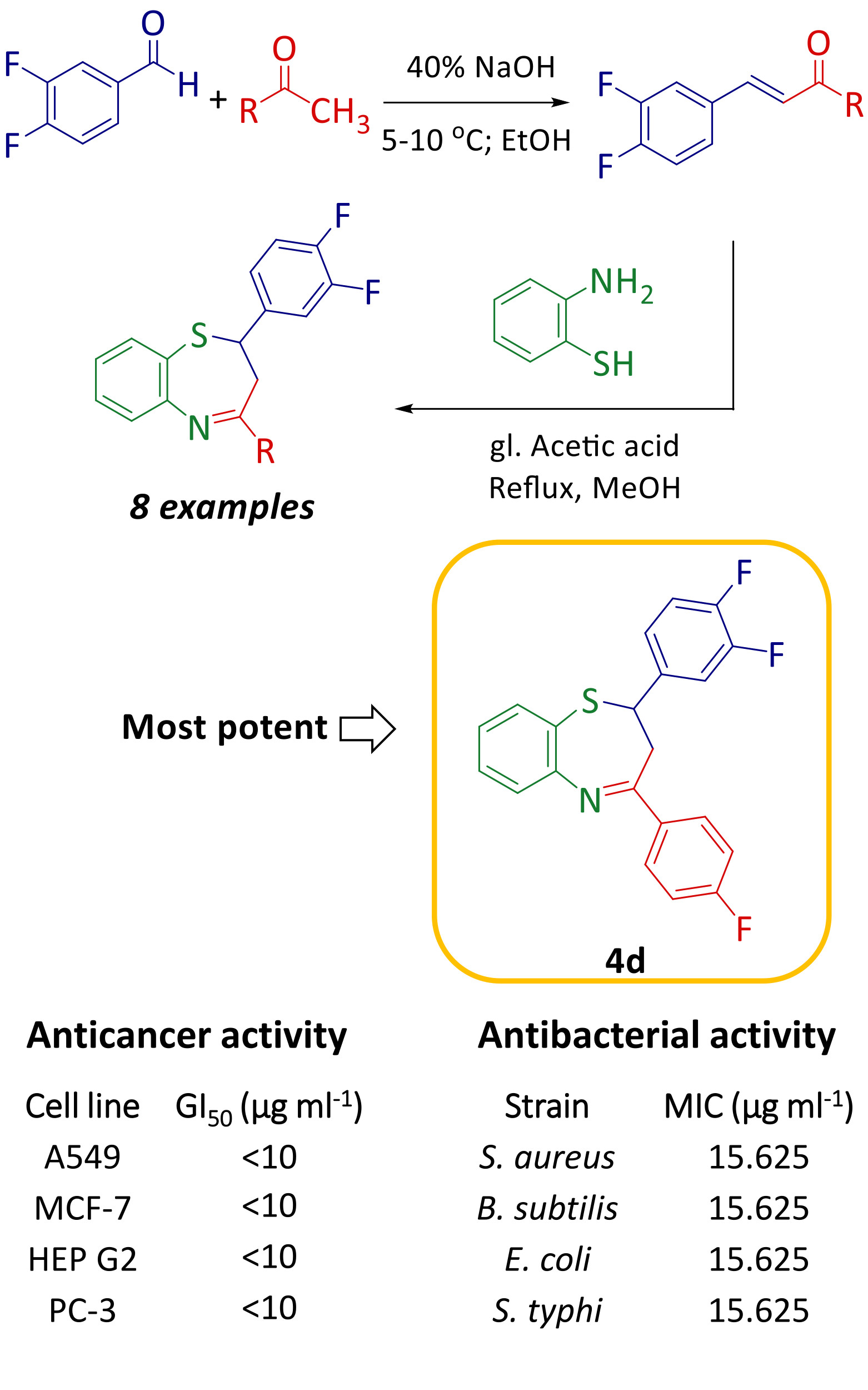
A series of novel fluorinated 1,5-benzothiazepine derivatives were synthesized, characterized and evaluated for in vitro anticancer and antibacterial activity. The in vitro anticancer activity of the synthesized compounds 4a–h was evaluated against four human cancer cell lines namely lung (A549), breast (MCF-7), liver (HEPG2) and prostate (PC-3). Compounds 4c, 4d, 4g and 4h exhibited good activity with GI50 <10 µg ml-1 against all four human cancer cell lines which was comparable to standard drug adriamycin. Additionally, antibacterial activity of synthesized compounds was estimated using Resazurin Microtiter Assay (REMA) and compared with standard drug ampicillin. Among the synthesized compounds, 4c, 4d, 4g and 4h showed good antibacterial activity and all the synthesized compounds were found to be more active towards gram negative than gram positive bacteria. These promising results obtained from in vitro anticancer and antibacterial activity, inferred that the synthesized compounds are capable of being anticancer as well as antibacterial agents.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray, CA. Cancer J. Clin. (2021) 1 (https://doi.org/10.3322/caac.21660)
A. Kamal, D. Dastagiri, M. Janaki Ramaiah, J. Surendranadha Reddy, E. Vijaya Bharathi, M. Kashi Reddy, M. Victor Prem Sagar, T. Lakshminarayan Reddy, S. N. C. V. L. Pushpavalli, M. Pal-Bhadra, Eur. J. Med. Chem. 46 (2011) 5817 (https://doi.org/10.1016/j.ejmech.2011.09.039)
B. Mansoori, A. Mohammadi, S. Davudian, S. Shirjang, B. Baradaran, Adv. Pharm. Bull. 7 (2017) 339 (https://doi.org/10.15171/apb.2017.041)
S. Bhat, S. Muthunatarajan, S. S. Mulki, K. Archana Bhat, K. H. Kotian, Int. J. Microbiol. 2021 (2021) (https://doi.org/10.1155/2021/8883700)
R. J. Fair, Y. Tor, Perspect. Medicin. Chem. (2014) 25 (https://doi.org/10.4137/PMC.S14459)
S. G. Jagadhani, S. G. Kundlikar, B. K. Karale, Orient. J. Chem. 31 (2015) 601 (https://doi.org/10.13005/ojc/310177)
F. L. Ansari, S. Umbreen, L. Hussain, T. Makhmoor, S. A. Nawaz, M. A. Lodhi, S. N. Khan, F. Shaheen, M. I. Choudhary, Atta-ur-Rahman, Chem. Biodivers. 2 (2005) 487 (https://doi.org/10.1002/cbdv.200590029)
A. B. Shaik, P. R. Yejella, S. Nissankararao, S. Shahanaaz, Anticancer. Agents Med. Chem. 20 (2020) 1115 (https://doi.org/10.2174/1871520620666200130091142)
K. L. Ameta, N. S. Rathore, B. Kumar, J. Serb. Chem. Soc. 77 (2012) 725 (https://doi.org/10.2298/JSC110715219А)
A. Sharma, G. Singh, A. Yadav, L. Prakash, Molecules 2 (1997) 129 (https://doi.org/10.3390/20900129)
V. R. Vutla, R.P. Yejella, R. Nadendla Int. J. Pharm. Sci. Res. 5 (2014) 453 (http://dx.doi.org/10.13040/IJPSR.0975-8232.5(2).453-62)
M. Mostofi, G. Mohammadi Ziarani, N. Lashgari, Bioorganic Med. Chem. 26 (2018) 3076 (https://doi.org/10.1016/j.bmc.2018.02.049)
G. Singh, N. Kumar, A. K. Yadav, A. K. Mishra, Heteroat. Chem. 13 (2002) 620 (https://doi.org/10.1002/hc.10051)
B. V. Kendre, M. G. Landge, S. R. Bhusare, Arab. J. Chem. 12 (2019) 2091 (https://doi.org/10.1016/j.arabjc.2015.01.007)
G. De Sarro, A. Chimirri, A. De Sarro, R. Gitto, S. Grasso, M. Zappalà, Eur. J. Med. Chem. 30 (1995) 925 (https://doi.org/10.1016/0223-5234(96)88311-5)
R. Di Santo, R. Costi, Farmaco 60 (2005) 385 (https://doi.org/10.1016/j.farmac.2005.03.006)
G. Grandolini, L. Perioli, V. Ambrogi, Eur. J. Med. Chem. 34 (1999) 701 (https://doi.org/10.1016/S0223-5234(99)00223-8)
S. A. Nawaz, S. Umbreen, A. Kahlid, F. L. Ansari, M. I. Choudhary, J. Enzyme Inhib. Med. Chem. 23 (2008) 206–212 (https://doi.org/10.1080/14756360701533080)
F. L. Ansari, F. Iftikhar, Ihsan-ul-Haq, B. Mirza, M. Baseer, U. Rashid, Bioorg. Med. Chem. 16 (2008) 7691 (https://doi.org/10.1016/j.bmc.2008.07.009)
D. M. Lokeshwari, N. D. Rekha, B. Srinivasan, H. K. Vivek, A. K Kariyappa, Bioorg. Med. Chem. Lett. 27 (2017) 3048 (https://doi.org/10.1016/j.bmcl.2017.05.059)
N. C. Desai, H. V. Vaghani, B. Y. Patel, T. J. Karkar, Ind. J. Pharm. Sci. 80 (2018) 242 (https://doi.org/10.4172/pharmaceutical-sciences.1000351)
H. J. Böhm, D. Banner, S. Bendels, M. Kansy, B. Kuhn, K. Müller, U. Obst-Sander, M. Stahl, ChemBioChem 5 (2004) 637 (https://doi.org/10.1002/cbic.200301023)
P. M. M. C. Rao, S. A. Rahaman, P. R. Yejella, Asian J. Pharm. Anal. Med. Chem. 4 (2016) 175
M. Upreti, S. Pant, A. Dandia, U. C. Pant, Phosphorus Sulfur Silicon Relat. Elem. 113 (1996) 165 (https://doi.org/10.1080/10426509608046387)
A. Dandia, M. Sati, A. Loupy, Green Chem. 4 (2002) 599 (https://doi.org/10.1039/b207004a)
H. Suwito, Jumina, Mustofa, P. Pudjiastuti, M. Z. Fanani, Y. Kimata-Ariga, R. Katahira, T. Kawakami, T. Fujiwara, T. Hase, H. M. Sirat, N. N. T. Puspaningsih, Molecules 19 (2014) 21473 (https://doi.org/10.3390/molecules191221473)
V. Vichai, K. Kirtikara, Nat. Protoc. 1 (2006) 1112 (https://doi.org/10.1038/nprot.2006.179)
S. F. Shaikh, P. P. Dhavan, P. R. Singh, S. P. Vaidya, B. L. Jadhav, M. M. V. Ramana Russ. J. Bioorg. Chem. 47 (2021) 571 (https://doi.org/10.1134/S1068162021020242).