Statistical optimization of lipase production from oil mill effluent by Acinetobacter sp. KSPE71 Scientific paper
Main Article Content
Abstract
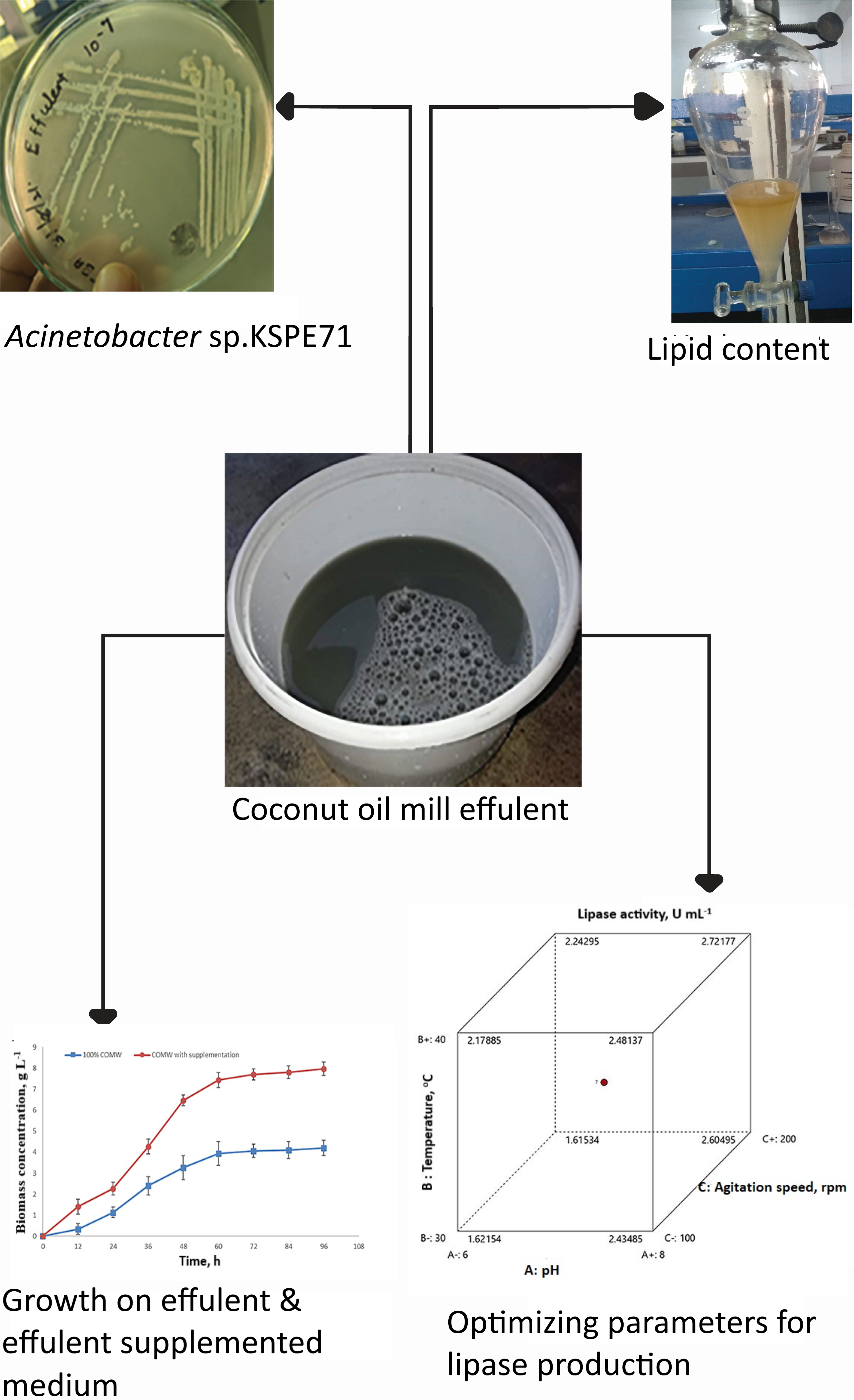
The present study investigated the valorisation of oil-rich residues of coconut oil mill effluent (COME) as a potential growth medium for the microbial production of extracellular lipase. The bacterial species isolated from oil mill effluent, Acinetobacter sp. KSPE71 was tested for its efficiency to grow and produce lipase in undiluted COME and 0.2 % yeast extract and 0.2 % NH4Cl supplemented COME. In this connection, the process parameters such as pH, temperature, agitation speed, and inoculum size were optimized to maximize the production using a central composite design in the Response surface methodology. At the optimized state of pH 7.5, 35 °C, 150 rpm with 0.6 % inoculum size, a maximum of 3.95 U mL-1 activity was obtained, four-fold higher than the basal condition. At this stage, 73 % of the lipid content was degraded. The present work results imply that the oil mill effluent can be used as a cheaper production medium for lipase and the new isolate Acinetobacter sp. KSPE71 as a potential lipase producer. The degradation of oil waste along with the production of the valuable product has multiple advantages of cost reduction of lipase and environmental concern.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. E. C. de Souza, B. D. Ribeiro, M. A. Z. Coelho, Appl. Biochem. Biotechnol. 189 (2019) 933 (https://doi.org/10.1007/s12010-019-03047-5)
C. Bernal, F. Guzman, A. Illanes, L. Wilson, Food Chem. 239 (2018) 189 (https://doi.org/10.1016/j.foodchem.2017.06.105)
R. Balakrishnaraja, V. Nisha, S. Geethadevi, C. Monisha, S. Devi, M. Pravinkumar, J. Revathi, K. Selvapriya, J. Adv. Chem. 12 (2016) 5010 (https://rajpub.com/index.php/jac/article/view/1052)
L. C. Phukon, R. Chourasia, M. Kumari, T. K. Godan, D. Sahoo, B. Parameswaran, A. K. Rai, Bioresour. Technol. 309 (2020) 123352 (https://doi.org/10.1016/J.BIORTECH.2020.123352)
E. Vanleeuw, S. Winderickx, K. Thevissen, B. Lagrain, M. Dusselier, B. P. A. Cammue, B. F. Sels, ACS Sustainable Chem. Eng. 7 (2019) 15828 (https://doi.org/10.1021/acssuschemeng.9b03257)
G. B. Pinto, F. M. L. Mendes, A. M. de S. Antunes, Mini-Rev. Org. Chem. 17 (2020) 701 (https://doi.org/10.2174/1570193X16666190913181530)
F. J. Contesini, M. G. Davanço, G. P. Borin, K. G. Vanegas, J. P. G. Cirino, R. R. de Melo, U. H. Mortensen, K. Hildén, D. R. Campos, P. de O. Carvalho, Catalysts 10 (2020) 1032 (https://doi.org/10.3390/catal10091032)
A. Sharma, S. Balda, N. Gupta, N. Capalash, P. Sharma, J. Cleaner Prod. 271 (2020) 122573 (https://doi.org/10.1016/J.JCLEPRO.2020.122573)
S. Hama, H. Noda, A. Kondo, Curr. Opin. Biotechnol. 50 (2018) 57 (https://doi.org/10.1016/J.COPBIO.2017.11.001)
F. L. C. Almeida, B. M. Travália, I. S. Gonçalves, M. B. S. Forte, Biofuels, Bioprod. Biorefin. 15 (2021) 1141 (https://doi.org/10.1002/BBB.2183)
Z. Ning, J. Ji, Y. He, Y. Huang, G. Liu, C. Chen, Energy Fuels 30 (2016) 7326 (https://doi.org/10.1021/acs.energyfuels.6b01097)
C. H. Okino-Delgado, D. Z. do Prado, R. Facanali, M. M. O. Marques, A. S. Nascimento, C. J. da C. Fernandes, W. F. Zambuzzi, L. F. Fleuri, PLoS One 12 (2017) e0186246 (https://doi.org/10.1371/JOURNAL.PONE.0186246)
N. Sarmah, D. Revathi, G. Sheelu, K. Y. Rani, S. Sridhar, V. Mehtab, C. Sumana, Biotechnol. Prog. 34 (2017) 5 (https://doi.org/10.1002/BTPR.2581)
X. Li, N. Shimizu, E. R. Rene, M. C. Veiga, Fermentation 7 (2021) 284 (https://doi.org/10.3390/FERMENTATION7040284)
Markets and Markets, https://www.marketsandmarkets.com/PressReleases/microbial-lipase.asp (accessed October 24, 2021)
M. Lopes, S. M. Miranda, I. Belo, Crit. Rev. Environ. Sci. Technol. 50 (2020) 2583 (https://doi.org/10.1080/10643389.2019.1704602)
T. Ahmad, T. Belwal, L. Li, S. Ramola, R. M. Aadil, Abdullah, Y. Xu, L. Zisheng, Trends Food Sci. Technol. 99 (2020) 21 (https://doi.org/10.1016/j.tifs.2020.02.017)
H. Kamilah, A. Al-Gheethi, T. A. Yang, K. Sudesh, Arab. J. Sci. Eng. 43 (2018) 3453 (https://doi.org/10.1007/s13369-018-3118-1)
P. Carlozzi, M. Seggiani, A. Capperucci, D. Tanini, P. Cinelli, A. Lazzeri, J. Biotechnol. 295 (2019) 28 (https://doi.org/10.1016/j.jbiotec.2019.02.006)
T. Szymczak, J. Cybulska, M. Podleśny, M. Frąc, Agriculture 11 (2021) 540 (https://doi.org/10.3390/agriculture11060540)
M. Lopes, S. M. Miranda, J. M. Alves, A. S. Pereira, I. Belo, Eur. J. Lipid Sci. Technol. 121 (2019) 1800188 (https://doi.org/10.1002/ejlt.201800188)
R. K. Sahoo, A. Das, M. Gaur, A. Sahu, S. Sahoo, S. Dey, P. K. S. M. Rahman, E. Subudhi, Prep. Biochem. Biotechnol. 50 (2020) 578 (https://doi.org/10.1080/10826068.2020.1719513)
S. Steudler, A. Werner, T. Walther, Solid State Fermentation, Advances in Biochemical Engineering/Biotechnology, Springer, Switzerland AG, Cham, 2019, pp. 51–81 (https://doi.org/10.1007/10_2019_85)
O. A. S. Moftah, S. Z. Grbavcic, W. A. S. Moftah, N. D. Lukovic, O. L. Prodanovic, S. M. Jakovetic, Z. D. Kneževic-Jugovic, J. Serb. Chem. Soc. 78 (2013) 781 (https://doi.org/10.2298/JSC120905005M)
V. Salgado, C. Fonseca, T. Lopes da Silva, J. C. Roseiro, A. Eusébio, Waste Biomass Valorization 11 (2020) 3207 (https://doi.org/10.1007/S12649-019-00725-7)
M. Lopes, C. Araújo, M. Aguedo, N. Gomes, C. Gonçalves, J. A. Teixeira, I. Belo, J. Chem. Technol. Biotechnol. 84 (2009) 533 (https://doi.org/10.1002/JCTB.2075)
P. Kanmani, K. Kumaresan, J. Aravind, Electron. J. Biotechnol. 18 (2015) 20 (https://doi.org/10.1016/j.ejbt.2014.11.003)
A. Eaton, Standard Methods for the Examination of Water and Wastewater, 21st ed., APHA-AWWA-WEF, Washington DC, 2005
G. L. Miller, Anal. Chem. 31 (2002) 426 (https://doi.org/10.1021/ac60147a030)
O. H. Lowry, N. J. Rosebrough, A. L. Farr, R. J. Randall, J. Biol. Chem. 193 (1951) 265 (https://pubmed.ncbi.nlm.nih.gov/14907713/)
U. K. Winkler, M. Stuckmann, J. Bacteriol. 138 (1979) 663 (https://doi.org/10.1128/jb.138.3.663-670.1979)
P. Gururaj, S. Ramalingam, G. Nandhini Devi, P. Gautam, Braz. J. Microbiol. 47 (2016) 647 (https://doi.org/10.1016/j.bjm.2015.04.002)
M. O. Al-Limoun, K. M. Khleifat, K. Y. Alsharafa, H. N. Qaralleh, S. A. Alrawashdeh, Biocatal. Biotransform. 37 (2019) 139 (https://doi.org/10.1080/10242422.2018.1506445)
M. O. Allimoun, M. R. Ananzeh, K. M. Khleifat, Int. J. Biosci. 6 (2015) 44 (https://doi.org/10.12692/ijb/6.10.44-56)
R. Patel, V. Prajapati, U. Trivedi, K. Patel, 3 Biotech 10 (2020) 508 (https://doi.org/10.1007/S13205-020-02501-0)
A. Isiaka Adetunji, A. Olufolahan Olaniran, Biotechnol. Biotechnol. Equip. 32 (2018) (https://doi.org/10.1080/13102818.2018.1514985)
A. D’Annibale, G. G. Sermanni, F. Federici, M. Petruccioli, Bioresour. Technol. 97 (2006) 1828 (https://doi.org/10.1016/J.BIORTECH.2005.09.001).