Comparison of different types of molar volume equations for the validity and applicability in a ternary carbamazepine + alizarin + methanol solution system and study of the corresponding molecular interactions Scientific paper
Main Article Content
Abstract
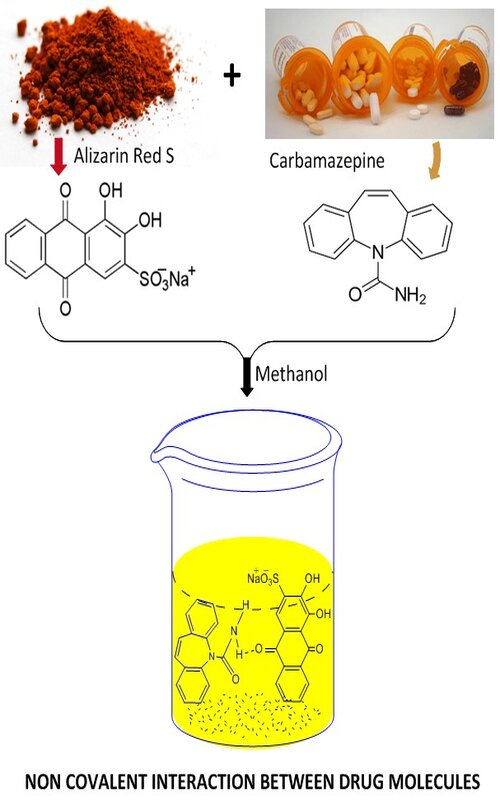
In this work, the molecular interaction between the carbamazepine and alizarin in methanol has been represented in terms of limiting apparent molar volumes and viscosity coefficients. Before further proceeding, the validity and applicability of the calculation of apparent molar volumes have also been checked by considering the available five types of frequently used equations, where the required modifications have proposed by the addition of hypothetical mass and concentration of the solute. After that, the limiting apparent molar volume and viscosity coefficients have been calculated using Masson equation and Jones–Dole equation respectively to predict and cross-check the interactions occurring between the molecules in ternary system. The equation marked with (1) has been found the best-fit equation, and the carbamazepine and alizarin in methanol are strongly bound (fVo = 23104 m3 mol-1 and B = 18.10 kg mol-1) to each other at the concentration 0.003 mol kg-1. The results have been interpreted in favour of the solute–cosolute interactions, which is dominant over the solute–solute and cosolute–cosolute interactions. The interpretations have been discussed with the help of intermolecular forces and non-covalent interactions.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. Fotia, S. Avnet, D. Granchi, N. Baldini, J. Orthop. Res. 30 (2012) 1486 (https://doi.org/10.1002/jor.22101.org/10.1002/jor.22101)
A. Saadia, I. Soliman, D. Louis, Eur. J. Pharm. Biopharm. 69 (2008) 342 (https://doi.org/10.1016/j.ejpb.2007.09.003)
K. Quliti, Neurosciences (Riyadh) 20 (2015) 107 (https://doi.org/10.17712/nsj.2015.2.20140501)
S. Qiu, A. Lai, M. Guo, K. Wang, X. Lai, U. Desai, N. Juma, M. Li, Cryst. Eng. Comm. 18 (2016) 2664 (https://doi.org/10.1039/C6CE00263C)
R. Andreozzi, M. Raffaele, M. Nicklas, Chemosphere 50 (2003) 1319 (https://doi.org/10.1016/s0045-6535(02)00769-5)
Q. Omar, J. Jaubert, J. Awan, Int. J. Chem. Eng. 1 (2018) 1 (https://doi.org/10.1155/2018/8689534)
I. Mohammad, R. E. Verrall, Canad. J. Chem. 67 (1989) 727 (https://doi.org/10.1139/v89-111)
M. Xiangyu, M. Felix , H. Siyuan, L. Michael, L. Xu, S. Rebecca, R. О. Wiliams III, Pharmaceutics 12 (2020) 379 (https://doi.org/10.3390/pharmaceutics12040379)
D. P. Shoemaker, C. W. Garland, Experiments in Physical Chemistry, McGraw-Hill Book Comp., New York, 1962 (https://doi.org/10.1002/ange.19630751334)
J. Wawer, J. Krakowiak, W. Grzybkowski, J. Chem. Thermodyn. 40 (2008) 1193 (https://doi.org/10.1016/j.jct.2008.04.008)
A. Nikumbh, R. Bhujbal, Int. J. Sci. Eng. Res. 5 (2014) 138 (https://www.ijser.org/onlineResearchPaperViewer.aspx?Apparent-molar-volumes-and-viscosity-B-coefficients-of-sodium.pdf)
M. Shakeel, K. Mahmood, J. Chin. Chem. Soc. 67 (2020) 1552 (https://doi.org/10.1002/jccs.202000128)
P. Amutha, X. Rajkumar, Asian. J. Chem. 23 (2011) 1360 (https://asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=23_3_97)
P. Muhuri, B. Das, D. Hasra, Ind. J. Chem. 35 (1996) 288 (http://nopr.niscair.res.in/handle/123456789/41321)
D. O. Masson, London, Edinburgh Dublin Phil. Mag. J. Sci. 8 (1929) 218 (https://doi.org/10.1080/14786440808564880)
Y. Marcus, J. Chem. Eng. Data 57 (2012) 617 (https://doi.org/10.1021/je201209h)
M. N. Roy, S. Choudhury, D. Ekka, Ind. J. Adv. Chemi. Sci. 6 (2018) 59 (https://doi.org/10.22607/IJACS.2018.602001)
E. Johnson, S. Keinan, P. Mori-Sanchez, J. Cohen, W. Yang, J. Am. Chem. Soc. 132 (2010) 6498 (https://doi.org/10.1021/ja100936w).