DBUH+I3 complex an efficient catalyst for the synthesis of 2-phenyl benzimidazole and benzothiazole derivatives
Main Article Content
Abstract
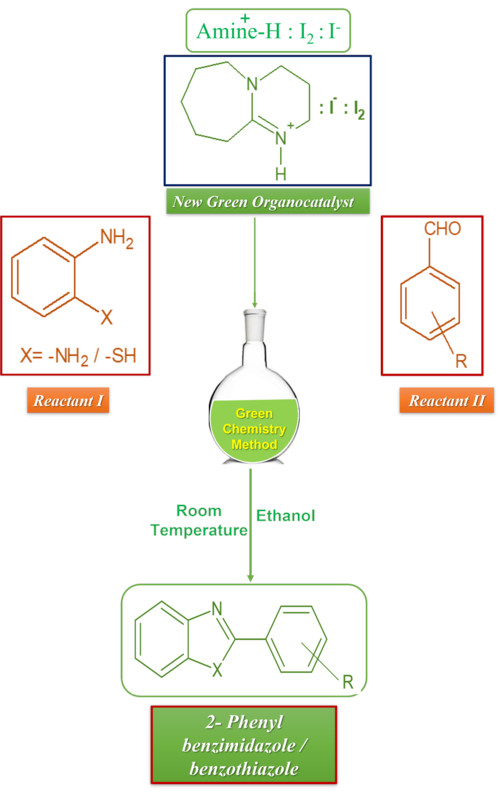
Herein, we have reported the facile synthesis of various benzimidazole/benzothiazole by using DBU–iodine–iodide as a green and simple catalyst. The R3NHI3 complexes have been formed by reacting an aqueous mixture of ammonium iodide and molecular iodine with the aqueous solution of amine. The structure of R3NHI3 complexes has been confirmed by spectroscopic techniques. The prepared amine–iodine complexes were screened as a catalysts in the synthesis of benzimidazole/benzothiazoles. Among the screened catalysts DBUHI3 complex has been found as most efficient catalyst. The synthesis of benzimidazoles and benzothiazoles has been achieved with the reaction of o-phenylene diamine/o-aminothiophenol and various substituted aryl aldehyde using DBUHI3 as a catalyst. The present protocol has offered some advantages over other reported protocols such as the mild reaction condition, commercially available precursors, inexpensive catalyst, short reaction time, the broad scope of the substrate, high yield, simple isolation of the product and environmentally benign method.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Velk, V. Baliharov, J. Fink-Gremmels, S. Bull, J. Lamka, L. Sklov, Res. Vet. Sci. 76 (2004) 95 (https://doi.org/10.1016/j.rvsc.2003.08.005)
C. D. Hadole, J. D. Rajput, R. S. Bendre, Org. Chem. Curr. Res. 7 (2018) 195 (https://doi.org/10.4172/2161-0401.1000195)
P. C. Sharmal, A. Sinhmar, A. Sharma, H. Rajak , D.P. Pathak, J. Enzym. Inhib. Med. Chem. 28 (2013) 240 (https://doi.org/10.3109/14756366.2012.720572)
V. S. Padalkar, B. N. Borse, V. D. Gupta, K. R. Phatangare, V. S. Patil, P. G. Umape, N. Sekar, Arabian J. Chem. 9 (2016) 1125 (https://doi.org/10.1016/j.arabjc.2011.12.006)
M. A. Abdelgawad, R. B. Bakr, H. A. Omar, Bioorg. Chem. 74 (2017) 82 (https://doi.org/10.1016/j.bioorg.2017.07.007)
I. Roberta, C. Antonio, M. Silvia, C. Paola, S. Gabriele, P. Sandra, S. Simona, L. Roberta, S. Giuseppina, Viruses 13 (2021) 58 (https://doi.org/10.3390/v13010058)
S. Noel, S. Cadet, E. Gras, C. Hureau, Chem. Soc. Rev. 42 (2013) 7747 (https://doi.org/10.1039/C3CS60086F)
Y. Gao, W. Xu, H. Ma, A. Obolda, W. Yan, S. Dong, M. Zhang, F. Li, Chem. Mater. 29 (2017) 6733 (https://doi.org/10.1021/acs.chemmater.7b01521)
G. Singh, H. K. Sahota, Plant Physiol. Biochem. 132 (2018) 166 (https://doi.org/10.1016/j.plaphy.2018.09.001)
M. Faheem, Anjali Rathaur, A. Pandey, V. K. Singh, A. K. Tiwari, Chem. Select. 5 (2020) 3981 https://doi.org/10.1002/slct.201904832
X. Gao, J. Liu, X. Zuo, X. Feng, Y. Gao, Molecules 25 (2020) 1675 (https://doi.org/10.3390/molecules25071675)
Zhan- Zhan-Hui Zhang, Liang Yin, Yong-Mei Wang, Catal. Commun. 8 (2007) 1126 (http://dx.doi.org/10.1016/j.catcom.2006.10.022)
Rui Wang, Xiao-xia Lu, Xiao-qi Yu, Lin Shi, Yong Sun, J. Mol. Catal., A 266 (2007) 198 (https://doi.org/10.1016/j.molcata.2006.04.071)
S. Rostamizadeh, M. Nojavan, F. Heshmatpoor, Heterocycl. Commun. 13 (2007) 305 (https://doi.org/10.1515/HC.2007.13.5.305)
F. Abdellaoui, C. Youssef, H. Ben Ammar, T. Roisnel, J. F. Soule, H. Doucet, ACS Catal. 6 (2016) 4248 (https://doi.org/10.1021/acscatal.6b00678)
P.R. Fernandes, P. Patil R. Shete, J. Chem. Rev. 4 (2022) 25 (https://dx.doi.org/10.22034/jcr.2022.316076.1132)
M. Bharathi, S. Indira, G. Vinoth, T. Mahalakshmi, E. Induja, K. Shamuga Bharathi, J. Coord. Chem. 73 (2020) 1 (http://dx.doi.org/10.1080/00958972.2020.1730335)
S. Majumdar, M. Chakraborty, N. Pramanikb, D. K. Maiti, RSC Adv. 5 (2015) 51012 (https://doi.org/10.1039/C5RA08183A)
T. T. Nguyen, X.-T. T. Nguyen, T.-L. H. Nguyen, P. H. Tran, ACS Omega 4 (2019) 368 (https://doi.org/10.1021/acsomega.8b02932)
M. A. Tzani, C. Gabriel, I. N. Lykakis, Nanomaterials 10 (2020) 2405 (https://doi.org/10.3390/nano10122405)
K. B. Rasal, Ganapati D. Yadav, Catal. Today (2017) 309 (http://dx.doi.org/10.1016/j.cattod.2017.10.014)
S. Singhal, P.j Khanna, S. S. Panda, and L. Khanna, J. Heterocycl. Chem. 56 (2016) 2702 (https://doi.org/10.1002/jhet.3649)
J. Kovvuri, B. Nagaraju, A. Kamal, A. K. Srivastava, ACS Comb. Sci. 18 (2016) 644 (https://doi.org/10.1021/acscombsci.6b00107)
N. H. Cano, J. G. Uranga, M. Nardi, A. Procopio, D. A. Wunderlin, and A. N. Santiago, Beilstein J. Org. Chem. 12 (2016) 2410 (https://doi.org/10.3762/bjoc.12.235)
S. Bonacci, G. Iriti, S. Mancuso, P. Novelli, R. Paonessa, S. Tallarico, and M. Nardi Catalysts 10 (2020) 845 (https://doi.org/10.3390/catal10080845)
M. L. Di Gioia, R. Cassano, P. Costanzo, N. H. Cano, L. Maiuolo, M. Nardi, F. P. Nicoletta, M. Oliverio, A. Procopio, Molecules 24 (2019) 2885 (https://doi.org/10.3390/molecules24162885)
N. P. Prajapati, R. H. Vekariya, M. A. Borad, H. D. Patel, RSC Adv. 4 (2014) 60176 (https://doi.org/10.1039/C4RA07437H)
J. J. Koenig, M. Breugst, in Catalysis by Molecular Iodine, H. Stefan, Ed., Wiley-VCH Publication, Weinheim, 2021, p. 233 https://doi.org/10.1002/9783527825738.ch7
S. Samanta, S. Mondal, Asian J. Org. Chem. 10 (2021) 2503 (https://doi.org/10.1002/ajoc.202100424)
L. C. R. M. da Frota, R. C. P. Canavez, S. L. da S. Gomes, P. R. R. Costa, A. J. M. da Silva, J. Braz. Chem. 20 (2009) 1916 (https://doi.org/10.1590/S0103-50532009001000021)
M. S. Refat, H. Al. Didamony, K. M. A. El-Nour, L. El-Zayat, J. Saudi Chem. Soc. 14 (2010) 323 (https://doi.org/10.1016/j.jscs.2010.04.004)
H. Naeimi, N. Alishahi, Org. Chem. Int. 2012 (2012) Article ID 498521 (https://doi.org/10.1155/2012/498521)
K. Gopalaiah, S. N. Chandrudu, RSC Adv. 5 (2015) 5015 (https://doi.org/10.1039/C4RA12490A)
S. V. Goswami, P. B. Thorat, V. N. Kadam, S. R. Bhusare, J. Chem. Biol. Phys. Sci. 1 (2011) 164 (https://www.researchgate.net/publication/269934184)
M. Malathi, P. S. Mohan, R. J. Butcher, C. K. Venil, Can. J. Chem. 87 (2009 1692 (https://doi.org/10.1139/V09-139)
C. Lia, H. Deng, T. Jin, Z. Liu, R. Jiang, C. Li, X. Jia, J. Li, Synthesis 49 (2017) 4350 (https://doi.org/10.1055/s-0036-1588487)
B. Maleki, H. Salehabadi, Eur. J. Chem. 1 (2010) 377 (https://doi.org/10.5155/eurjchem.1.4.377-380.165)
R. Bhata, S. Karhale, S. Ardeb, V. Helavia, Iranian J. Catal. 9 (2019) 173 (http://ijc.iaush.ac.ir/article_664816_76d3680235636f284a8f914d9421b93e.pdf)
M. Maphupha, W. P. Juma, C. B. de Koning, D. Brady, RSC Adv. 8 (2018) 39496 (https://doi.org/10.1039/C8RA07377E)
C. Praveen, A. Nandkumar, P. Dheenkuma, D. Muralidharan, P. T. Perumal, J. Chem. Sci. 124 (2012) 609 (https://doi.org/10.1007/s12039-012-0251-3)
Y. Han, X. Wang, X, Wang, L. Lv, G. Diao, Y. Yuan, Synthesis 44 (2012) 3027 (https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0032-1317035)
S. Ray, P. Das, B. Banerjee, A. Bhaumik, C. Mukhopadhyay, RSC Adv. 5 (2015) 72745 (http://dx.doi.org/10.1039/c5ra14894d)
W. Senapak, R. Saeeng, J. Jaratjaroonphong, U. Sirion, Mol. Catal. 458 (2018) 97 (https://doi.org/10.1016/j.mcat.2018.06.017).