Transformation of fluorite δ-Bi2O3 into a new tetragonal phase Scientific paper
Main Article Content
Abstract
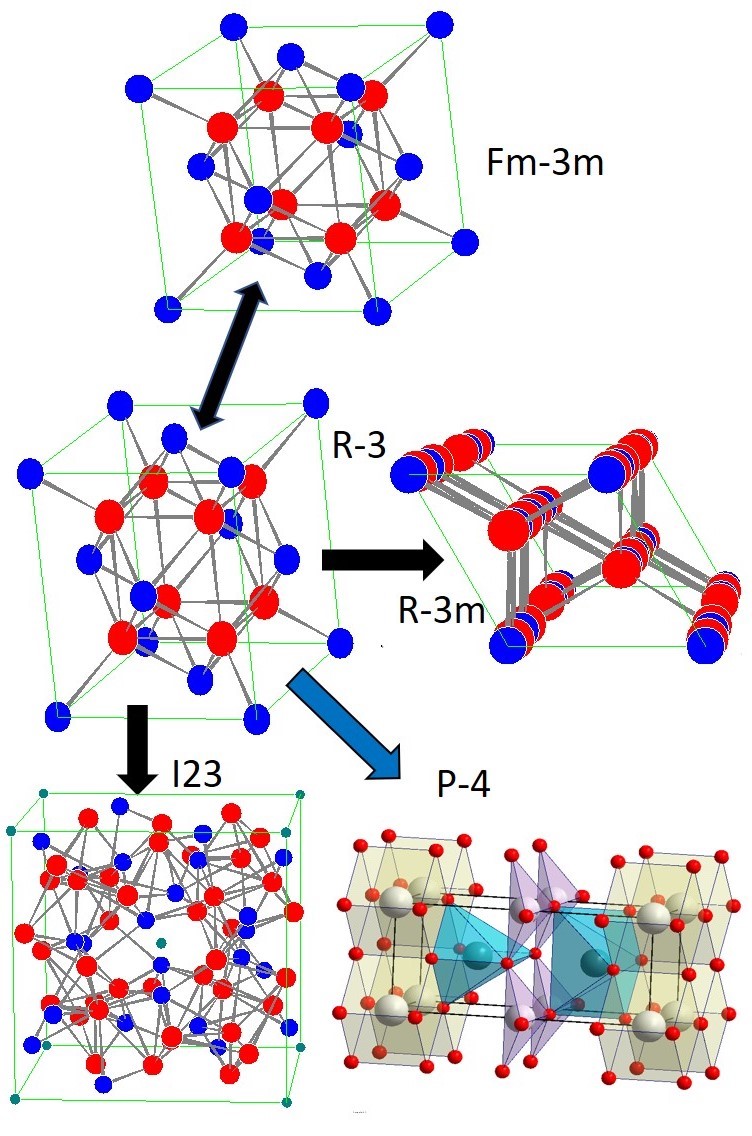
Bismuth oxide kinetically stabilized by doping with a metastable structure of disordered fluorite δ-Bi2O3 has a unique conductivity. Oxygen selective membranes at intermediate temperatures ~550 °C, on the base of cermet δ-Bi2O3/Ag, have the highest potential for air separation and can be used to produce oxygen for distributed multigeneration by burning fossil carbon fuels. When searching for the optimal composition of δ-Bi2O3, the degradation of fluorite into a new tetragonal phase was discovered in ceramics synthesized using mechanical activation. The tetragonal phase is formed and exists in a topotaxial composite with the fluorite structure. For a relatively stable over a wide temperature range tetragonal phase with a = 0.3854, c = 0.88905 nm, S.G. P-4, crystal structure and atomic coordinates have been proposed. In samples of fluorite and topotaxial composite, the Raman and Mössbauer spectra were recorded and discussed. The discovery of a new tetragonal phase of doped bismuth oxide and its existence area makes it possible to optimize the composition and the synthesis of a more stable solid electrolyte δ-Bi2O3 with high conductivity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Russian Foundation for Fundamental Investigations
Grant numbers 20-03-00349
References
H. A. Harwig, A.G. Gerards, J. Solid State Chem. 26 (1978) 265 (https://dx.doi.org/10.1016/0022-4596(78)90161-5)
P. Shuk, H. D. Wiemhofer, U. Guth, W. Gopel, M. Greenblatt, Solid State Ionics 89 (1996) 179 (https://dx.doi.org/10.1016/0167-2738(96)00348-7)
V. V. Kharton, F. M. B. Marques, A. Atkinson, Solid State Ionics 174 (2004) 135 (https://dx.doi.org/10.1016/j.ssi.2004.06.015)
B. Singh, S. Ghosh, S. Aich, B. Roy, J. Power Sources 339 (2017) 103 (https://dx.doi.org/10.1016/j.jpowsour.2016.11.019)
A. Dapčević, D. Poleti, L. Karanović, J. Miladinović, J. Serb. Chem. Soc. 82 (2017) 1433 (https://doi.org/10.2298/JSC170711111D)
A. Matsumoto, Y. Koyama, I. Tanaka, Phys. Rev., B 81 (2010) 094117 (https://dx.doi.org/10.1103/PhysRevB.81.094117)
N. Jiang, R. M. Buchanan, F. E. G. Henn, A. F. Marshall, D. A. Stevenson, E. D. Wachsman, Mater. Res. Bull. 29 (1994) 247 (https://doi.org/10.1016/0025-5408(94)90020-5)
S. Boyapati, E. D. Wachsman, N. Jiang, Solid State Ionics 140 (2001) 149 (https://doi.org/10.1016/S0167-2738(01)00698-1)
S. Boyapati, E. D. Wachsman, B. C. Chakoumakos, Solid State Ionics 138 (2001) 293 (https://doi.org/10.1016/s0167-2738(00)00792-x)
E. D. Wachsman, J. Eur. Ceram. Soc. 24 (2004) 1281 (http://dx.doi.org/10.1016/s0955-2219(03)00509-0)
V. V. Zyryanov, A. S. Ulihin, Ceram. Int. 48 (2022) 16877 (https://doi.org/10.1016/j.ceramint.2022.02.242)
V. V. Zyryanov, Inorg. Mater. 41 (2005) 378 (http://dx.doi.org/10.1007/s10789-005-0140-y)
V. V. Zyryanov, Russ. Chem. Rev. 77 (2008) 105 (https://doi.org/10.1070/RC2008v077n02ABEH003709)
V. V. Zyryanov, S. A. Petrov, A. S. Ulihin, Ceram. Int. 47 (2021) 29499 (https://doi.org/10.1016/j.ceramint.2021.07.118)
B.-H. Yun, C.-W. Lee, I. Jeong, K. T. Lee, Chem. Mater. 29 (2017) 10289 (https://doi.org/10.1021/acs.chemmater.7b03894)
A. J. Wright, J. Luo, J. Mater. Sci. 1000 (2020) 9812 (https://doi.org/10.1007/s10853-020-04583-w)
F. F. H. Aragon, J. C. R. Aquino, J. E. Ramos, J. A. H. Coaquira, I. Gonzalez, W. A. A. Macedo, S. W. da Silva, P. C. Morais, J. Appl. Phys. 122 (2017) 204302 (https://doi.org/10.1063/1.4999457)
R. Nedyalkova, D. Niznansky, A-C. Roger, Catal. Comm. 10 (2009) 1875 (http://dx.doi.org/10.1016/j.catcom.2009.06.017)
A. Kirsch, M. M. Murshed, F. J. Litterst, T. M. Gesing, J. Phys. Chem., C 123 (2019) 3161 (http://dx.doi.org/10.1021/acs.jpcc.8b09698)
F. D. Hardcastle, I. E. Wachs, J. Solid State Chem. 97 (199) 319 (https://doi.org/10.1016/0022-4596(92)90040-3).