Performance of carbon-coated magnetic nanocomposite in methylene blue and arsenate treatment from aqueous solution Scientific paper
Main Article Content
Abstract
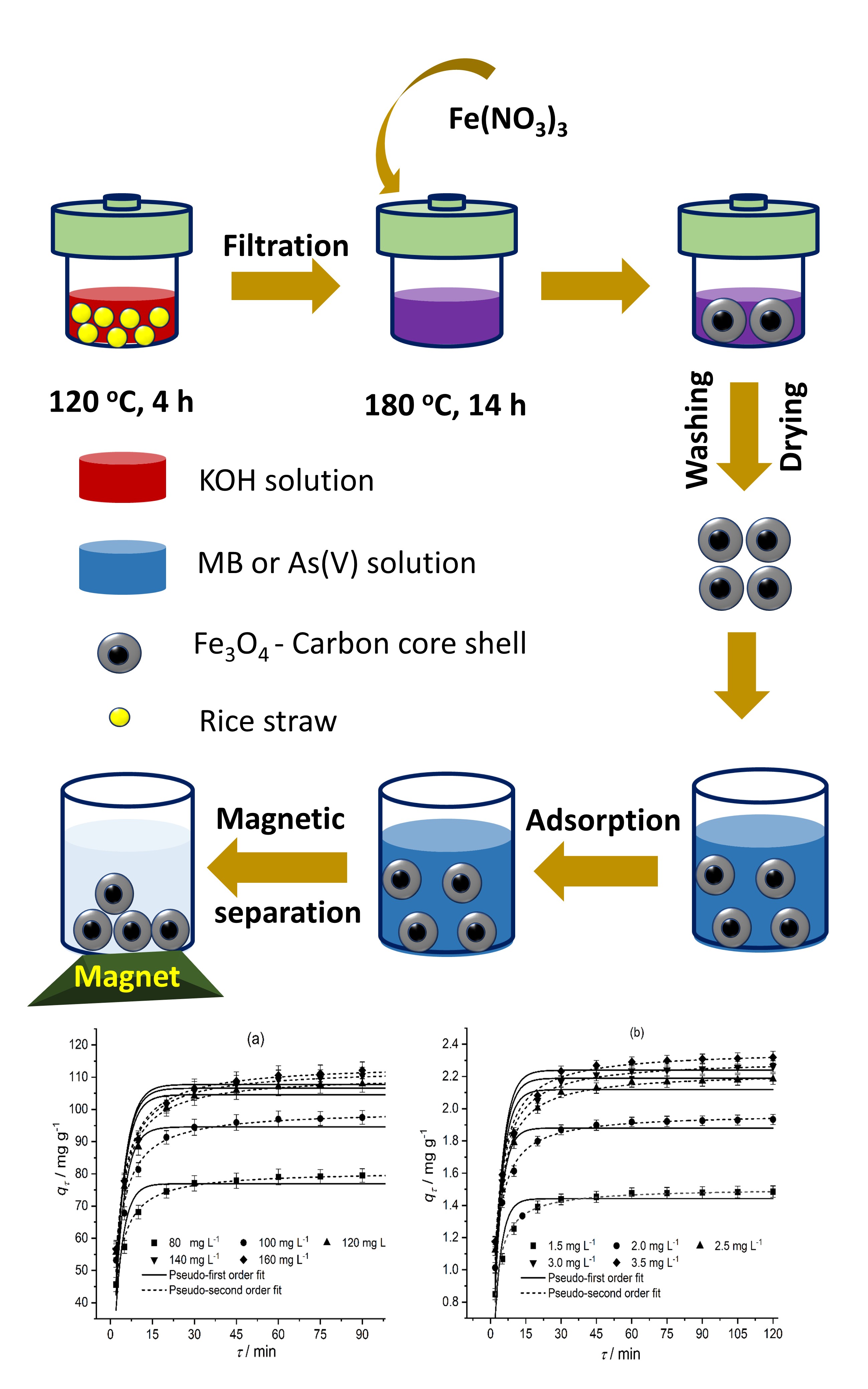
Herein, carbon-coated magnetic nanocomposite fabricated by a low-temperature hydrothermal method was used for methylene blue and arsenate treatment in aqueous solution. The Langmuir model fits the experimental data with a calculated maximum adsorption capacity of 110.63 and 2.31 mg g-1 for methylene blue and arsenate adsorption, respectively. Furthermore, the adsorption mechanisms of methylene blue as well as arsenate are physical adsorption and a combination of physical adsorption and chemisorption, respectively. Gibbs energy change with negative values indicates that methylene blue and arsenate adsorption on magnetic materials occurs naturally. This research demonstrated a simple, efficient, and reliable method for removing methylene blue and arsenate.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. Ji, C. Miao, H. Liu, L. Feng, X. Yang, H. Guo, Nanoscale Res. Lett. 13 (2018) 178 (https://doi.org/10.1186/s11671-018-2580-8)
W. J. Liu, K. Tian, H. Jiang, H. Q. Yu, Sci Rep 3 (2013) 2419 (https://doi.org/10.1080/19443994.2015.1132476)
T. H. Nguyen, T. H. Pham, H. T. N. Thi, T. N. Nguyen, M. V. Nguyen, T. T. Dinh, M. P. Nguyen, T. Q. Do, T. Phuong, T. T. Hoang, T. T. M. Hung, V. H. T. Thi, J. Chem. 2019 (2019) 1 (https://doi.org/10.1155/2019/5295610)
M. Inyang, B. Gao, P. Pullammanappallil, W. Ding, A. R. Zimmerman, Bioresour. Technol. 101 (2010) 8868 (https://doi.org/10.1016/j.biortech.2010.06.088)
N. Besharati, N. Alizadeh, S. Shariati, J. Mex. Chem. Soc. 62 (2018) 110 (https://doi.org/10.29356/jmcs.v62i3.433)
W. Chen, R. Parette, J. Zou, F. Cannon, B. Dempsey, Water Res. 41 (2007) 1851 (https://doi.org/10.1016/j.watres.2007.01.052)
M. Zhang, B. Gao, S. Varnoosfaderani, A. Hebard, Y. Yao, M. Inyang, Bioresour. Technol. 130 (2013) 457 (https://doi.org/10.1016/j.biortech.2012.11.132)
L. Huang, J. Cai, M. He, B. Chen, B. Hu, Ind. Eng. Chem. Res. 57 (2018) 6201 (https://doi.org/10.1021/acs.iecr.7b05294)
N. S. Pham, P. T. Q. Phan, B. N. Nguyen, V. X. Le, J. Appl. Electrochem. (2022) (https://doi.org/10.1007/s10800-022-01747-1)
N. S. Pham, V. X. Le, J. Electroanal. Chem. 921 (2022) 116507 (https://doi.org/10.1016/j.jelechem.2022.116507)
N. S. Pham, B. N. Nguyen, A. Q. K. Nguyen, J. Appl. Electrochem. (2022) (https://doi.org/10.1007/s10800-022-01784-w)
K. Dai, F. Wang, W. Jiang, Y. Chen, J. Mao, J. Bao, Nanoscale Res. Lett. 12 (2017) 528 (https://doi.org/10.1186/s11671-017-2295-2)
N. S. Pham, Y. H. Seo, E. Park, T. D. D. Nguyen, I.-S. Shin, Data Br. 31 (2020) 105891 (https://doi.org/10.1016/j.dib.2020.105891)
N. S. Pham, Y. H. Seo, E. Park, T. D. D. Nguyen, I.-S. Shin, Electrochim. Acta 353 (2020) 136446 (https://doi.org/10.1016/j.electacta.2020.136446)
V. X. Le, H. Lee, N. S. Pham, S. Bong, H. Oh, S.-H. Cho, I.-S. Shin, Sensors Actuators, B 346 (2021) 130552 (https://doi.org/10.1016/j.snb.2021.130552)
N. S. Pham, P. T. Q. Phan, V. X. Le, J. Appl. Electrochem. 52 (2022) 1343 (https://doi.org/10.1007/s10800-022-01716-8)
L. Zhu, F. Shen, R. L. Smith, L. Yan, L. Li, X. Qi, Chem. Eng. J. 316 (2017) 770 (https://doi.org/10.1016/j.cej.2017.02.034)
L. Ai, C. Zhang, Z. Chen, J. Hazard Mater. 192 (2011) 1515 (https://doi.org/10.1016/j.jhazmat.2011.10.041)
X. Bao, Z. Qiang, J.-H. Chang, W. Ben, J. Qu, J. Environ. Sci. 26 (2014) 962 (https://doi.org/10.1016/S1001-0742(13)60485-4)
L. Verma, M. A. Siddique, J. Singh, R. N. Bharagava, J. Environ. Manage. 250 (2019) 109452 (https://doi.org/10.1016/j.jenvman.2019.109452)
J. Wang, J. Xu, N. Wu, J. Exp. Nanosci. 12 (2017) 297 (https://doi.org/10.1080/17458080.2017.1325016)
B. Qiu, H. Gu, X. Yan, J. Guo, Y. Wang, D. Sun, Q. Wang, M. Khan, X. Zhang, B. L. Weeks, D. P. Young, Z. Guo, S. Wei, J. Mater. Chem. A 2 (2014) 17454 (https://doi.org/10.1039/C4TA04040F)
H. Zeng, W. Qi, L. Zhai, F. Wang, J. Zhang, D. Li, J. Environ. Chem. Eng. 9 (2021) 105951 (https://doi.org/10.1016/j.jece.2021.105951)
Y. Bulut, H. Aydın, Desalination 194 (2006) 259 (https://doi.org/10.1016/j.desal.2005.10.032)
K. Y. Foo, B. H. Hameed, Desalin. Water Treat. 19 (2012) 255 (https://doi.org/10.5004/dwt.2010.1214)
A. Sharma, N. Verma, A. Sharma, D. Deva, N. Sankararamakrishnan, Chem. Eng. Sci. 65 (2010) 3591 (https://doi.org/10.1016/j.ces.2010.02.052)
X. Shi, C. Wang, Y. Ma, H. Liu, S. Wu, Q. Shao, Z. He, L. Guo, T. Ding, Z. Guo, Powder Technol. 356 (2019) 726 (https://doi.org/10.1016/j.powtec.2019.09.002)
B. Gu, J. Schmitt, Z. Chen, L. Llang, J. F. McCarthy, Environ. Sci. Technol 28 (1994) 38 (https://doi.org/10.1021/es00050a007)
L. Ding, B. Zou, W. Gao, Q. Liu, Z. Wang, Y. Guo, X. Wang, Y. Liu, Colloids Surfaces, A 446 (2014) 1 (https://doi.org/10.1016/j.colsurfa.2014.01.030)
C. Li, Z. Xiong, J. Zhang, C. Wu, J. Chem. Eng. Data 60 (2015) 3414 (https://doi.org/10.1021/acs.jced.5b00692)
T. S. Anirudhan, J. Nima, S. Sandeep, V. R. N. Ratheesh, Chem. Eng. J. 209 (2012) 362 (https://doi.org/10.1016/j.cej.2012.07.129)
M. A. Ahmad, N. A. Ahmad Puad, O. S. Bello, Water Resour. Ind. 6 (2014) 18 (https://doi.org/10.1016/j.wri.2014.06.002)
X. Zhou, J. Zhou, Y. Liu, J. Guo, J. Ren, F. Zhou, Fuel 233 (2018) 469 (https://doi.org/10.1016/j.fuel.2018.06.075).