Natural flavonoids in Delonix regia leaf as an antimycobacterial agent: An in silico study Scientific paper
Main Article Content
Abstract
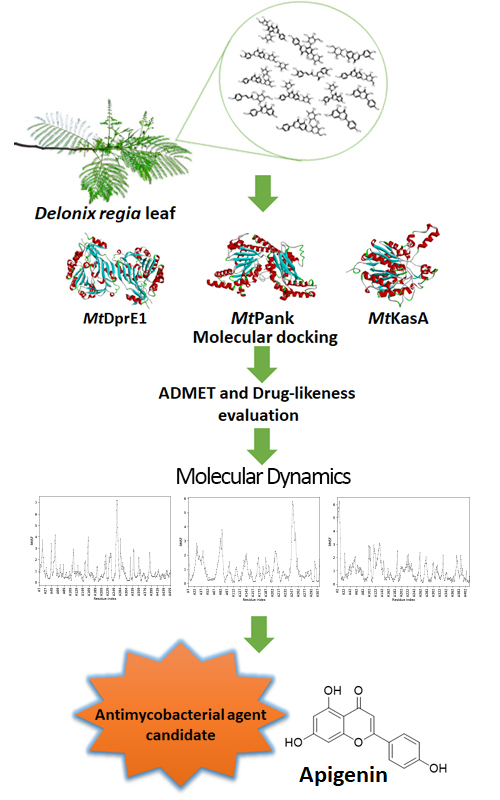
Multi-drug resistant (MDR) and extensively-drug resistant (XDR) as results of continuous use of antibiotics encourage the development of new antimycobacterial drugs. In this study, 13 flavonoid compounds from the flamboyant leaf plant were studied for their inhibitory properties of MtKasA, MtDprE and MtPank which are significant enzymes in Mycobacterium tuberculosis, as well as for their molecular docking, molecular dynamics and prediction of ADMET-drug likeness. The results of molecular docking studies revealed that compound F13 (apigenin) was the most potent compound because it was able to bind the most amino acids as indicated by the native ligand of each enzyme. Molecular dynamics studies showed that compound F13 forms a stable complex with MtKasA. The results of the ADMET-drug likeness analysis concluded that compound F13 was the most promising compound. Overall, compound F13 has the potential to be used as a treatment therapy against M. tuberculosis.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
World Health Organization, Global Tuberculosis Report 2021, https://reliefweb.int/report/world/global-tuberculosis-report-2021?gclid=CjwKCAiA_vKeBhAdEiwAFb_nrRsQtbL3Ty7CYBrt8PzmQTr0a4NFLirHj97ujmUL8brrzoMgEruwWxoCVmYQAvD_BwE (accessed 22th September 2022)
I. Rossi, R. Bettini, F. Buttini, Curr. Pharm. Des. 27 (2021) 1436 (https://doi.org/10.2174/1381612827666210122143214)
G. F. S. Fernandes, A. M. Thompson, D. Castagnolo, W. A. Denny, J. L. Dos Santos, J. Med. Chem. 65 (2022) 7489–7531 (https://doi.org/https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00227)
L. Pinzi, G. Rastelli, Int. J. Mol. Sci. 20 (2019) 4331 (https://doi.org/10.3390/IJMS20184331)
S. A. Mir, J. Pharm. Res. Int. 33 (2021) 278 (https://doi.org/10.9734/jpri/2021/v33i45b32805)
M. K. S. Siam, M. U. S. Shohan, Z. Zafroon, bioRxiv 4 (2020) (https://doi.org/10.1101/2020.04.28.067090)
S. M. Batt, T. Jabeen, V. Bhowruth, L. Quill, P. A. Lund, L. Eggeling, L. J. Alderwick, K. Fuẗterer, G. S. Besra, Proc. Natl. Acad. Sci. U.S.A. 109 (2012) 11354 (https://doi.org/10.1073/pnas.1205735109)
M. Brecik, I. Centárová, R. Mukherjee, G. S. Kolly, S. Huszár, A. Bobovská, E. Kilacsková, V. Mokošová, Z. Svetlíková, M. Šarkan, J. Neres, J. Korduláková, S. T. Cole, K. Mikušová, ACS Chem. Biol. 10 (2015) 1631 (https://doi.org/10.1021/acschembio.5b00237)
C. Bjorkelid, T. Bergfors, A. K. V. Raichurkar, K. Mukherjee, K. Malolanarasimhan, B. Bandodkar, T. A. Jones, J. Biol. Chem. 288 (2013) 18260 (https://doi.org/10.1074/jbc.M113.476473)
M. C. Martini, T. Zhang, J. T. Williams, R. B. Abramovitch, P. J. Weathers, S. S. Shell, J. Ethnopharmacol. 262 (2020) 113191 (https://doi.org/10.1016/J.JEP.2020.113191)
D. Das, S. Das, M. Pandey, D. Bhattacharyay, Eur. J. Med. Plants 31 (2020) 19 (https://doi.org/10.9734/ejmp/2020/v31i430226)
A. R. Elnaas, D. Grice, J. Han, Y. Feng, A. Di Capua, T. Mak, J. A. Laureanti, G. W. Buchko, P. J. Myler, G. Cook, R. J. Quinn, M. Liu, Molecules 25 (2020) 2384 (https://doi.org/10.3390/MOLECULES25102384)
E. Hernández-García, A. García, E. Garza-González, F. G. Avalos-Alanís, V. M. Rivas-Galindo, J. Rodríguez-Rodríguez, V. M. Alcantar-Rosales, C. Delgadillo-Puga, M. del Rayo Camacho-Corona, J. Ethnopharmacol. 230 (2019) 74 (https://doi.org/10.1016/J.JEP.2018.10.031)
M. Kumar, S. Prakash, Radha, N. Kumari, A. Pundir, S. Punia, V. Saurabh, P. Choudhary, S. Changan, S. Dhumal, P. C. Pradhan, O. Alajil, S. Singh, N. Sharma, T. Ilakiya, S. Singh, & M. Mekhemar, Antioxidants 10 (2021) 1061 (https://doi.org/10.3390/ANTIOX10071061)
A. A. Rabaan, S. Alhumaid, H. Albayat, M. Alsaeed, F. S. Alofi, M. H. Al-Howaidi, S. A. Turkistani, S. M. Alhajri, H. E. Alahmed, A. B. Alzahrani, M. M. Mashraqi, S. Alwarthan, M. Alhajri, F. S. Alshahrani, S. A. Almuthree, R. A. Alsubki, A. A. Abuzaid, M. Alfaresi, M. A. Al Fares, A. Al Mutair, Molecules 27 (2022) 1 (https://doi.org/10.3390/MOLECULES27165335)
P. K. Boniface, E. I. Ferreira, Stud. Nat. Prod. Chem. 65 (2020) 85 (https://doi.org/10.1016/B978-0-12-817905-5.00003-2)
A. Pawar, P. Jha, M. Chopra, U. Chaudhry, D. Saluja, Sci. Rep. 10 (2020) 1 (https://doi.org/10.1038/s41598-020-57658-8)
Y. Liu, A. R. Fernie, T. Tohge, Plants 11 (2022) 564 (https://doi.org/10.3390/PLANTS11040564)
H. El-gizawy, A. Alazzouni, A. El-haddad, Pharmacogn. Commun. 8 (2018) 125 (https://doi.org/https://doi.org/10.5530/pc.2018.3.26)
A. Abdou, A. M. M. Abdel-Mawgoud, Appl. Organomet. Chem. 36 (2022) e6600 (https://doi.org/10.1002/AOC.6600)
N. Nagasundaram, K. Padmasree, S. Santhosh, N. Vinoth, N. Sedhu, A. Lalitha, J. Mol. Struct. 1263 (2022) 133091 (https://doi.org/10.1016/j.molstruc.2022.133091)
D. E. V. Pires, T. L. Blundell, D. B. Ascher, J. Med. Chem. 58 (2015) 4066 (https://doi.org/https://pubs.acs.org/doi/10.1021/acs.jmedchem.5b00104)
A. Tripathi, V. Bankaitis, J. Mol. Med. Clin. Appl. 2 (2018) 1 (https://doi.org/10.16966/2575-0305.106)
Y. Yuniwati, M. F. R. Syaban, S. G. Anoraga, F. L. Sabila, Acta Inform. Medica 30 (2022) 91 (https://doi.org/10.5455/aim.2022.30.91-95)
S. Luckner, C. Machutta, P. Tonge, C. Kisker, Mol. Cell. Biochem. 17 (2009) 1004 (https://doi.org/10.1016/j.str.2009.04.012)
M. T. Ali, N. Blicharska, J. A. Shilpi, V. Seidel, Sci. Rep. 8 (2018) 1 (https://doi.org/10.1038/s41598-018-30209-y)
B. K. K. Reddy, S. Landge, S. Ravishankar, V. Patil, V. Shinde, S. Tantry, M. Kale, A. Raichurkar, S. Menasinakai, N. V. Mudugal, A. Ambady, A. Ghosh, R. Tunduguru, P. Kaur, R. Singh, N. Kumar, S. Bharath, A. Sundaram, J. Bhat, V. K. Sambandamurthy, C. Björkelid, T. A. Jones, K. Das, B. Bandodkar, K. Malolanarasimhan, K. Mukherjee, V. Ramachandran, Antimicrob. Agents Chemother. 58 (2014) 3312 (https://doi.org/10.1128/AAC.00140-14)
J. Chen, H. Yang, L. Zhu, Z. Wu, W. Li, Y. Tang, G. Liu, Chem. Res. Toxicol. 33 (2020) 640 (https://doi.org/10.1021/acs.chemrestox.9b00447)
D. Machado, M. Girardini, M. Viveiros, M. Pieroni, Front. Microbiol. 9 (2018) 1 (https://doi.org/10.3389/fmicb.2018.01367)
A. Abdou, H. M. Mostafa, A. M. M. Abdel-Mawgoud, Inorg. Chim. Acta 539 (2022) 121043 (https://doi.org/10.1016/J.ICA.2022.121043)
M. Jamroz, A. Kolinski, S. Kmiecik, Bioinformatics 30 (2014) 2150 (https://doi.org/10.1093/bioinformatics/btu184)
R. R. Renantha, A. R. Liga, C. B. Tanugroho, L. X. Denovian, S. L. Az, Z. Budiyanto, A. A. Parikesit, J. Pharm. Pharmacogn. Res. 10 (2022) 660 (https://doi.org/https://doi.org/10.56499/jppres22.1375_10.4.660).