Binding interactions of actinomycin D anticancer drug with bile salts micelles Scientific paper
Main Article Content
Abstract
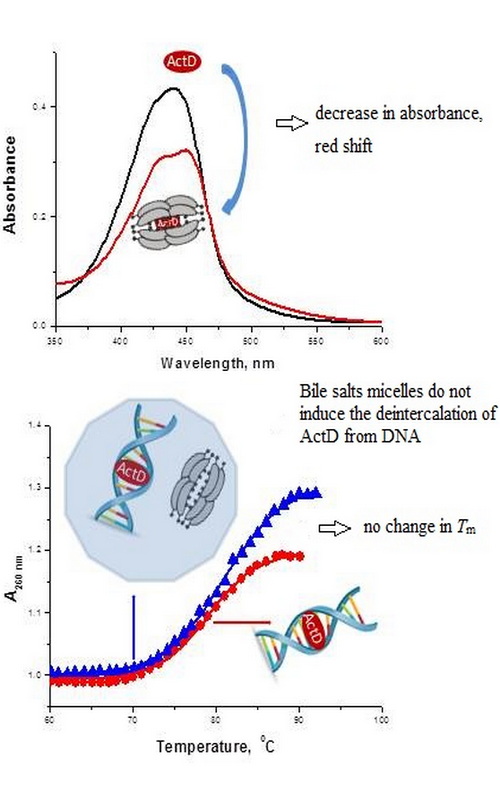
The interactions of actinomycin D (ActD) anticancer drug with two bile salts of different hydrophobicity (sodium cholate (NaC) and sodium deoxycolate (NaDC) and the influence of these bile salts aggregates on the ActD–DNA complex was investigated in 10 mM phosphate buffer (pH 7.4) by UV–Vis spectroscopy (absorption and thermal denaturation). The binding strength of ActD to NaDC is higher than for NaC, and this difference attests stronger hydrophobic interactions between ActD and NaDC micelles. Also, the partition coefficient is significantly higher for NaDC micelles than for NaC micelles, in line with larger aggregates formed by NaDC. The spectral profile of ActD molecules in NaC and NaDC micelles, in comparison with different solvents, implies that ActD molecule experiences a hydrophobic environment in bile salts aggregates. Regarding the influence of NaC and NaDC aggregates on the ActD–DNA complex, it was shown that the presence of both bile salts micelles do not induce the deintercalation of ActD molecules from DNA duplex.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. C. Schink, D. K. Singh, A.W. Rademaker, D. S. Miller, J. R. Lurain, Obstet. Gynecol. 80 (1992) 817 (https://doi.org/10.1016/0020-7292(93)90503-O)
H. Hosoi, Pediatr. Int. 58 (2016) 81 (https://doi.org/10.1111/ped.12867)
R. D. Jenkin, R. D. Jeffs, C. A. Stephens, M. J. Sonley, Can. Med. Assoc. J. 115 (1976) 136 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1878570/pdf/canmedaj01484-0048.pdf)
H. M. Sobell, Proc. Natl. Acad. Sci. U.S.A. 82 (1985) 5328 (https://doi.org/10.1073/pnas.82.16.5328)
А. F. Hofmann, L. R. Hagey, Cell Mol. Life Sci. 65 (2008) 2461 (https://doi.org/10.1007/s00018-008-7568-6)
J. Maldonado-Valderrama, P. Wilde, A. Macierzanka, A. Mackie, Adv. Colloid Interface Sci. 165 (2011) 36 (https://doi.org/10.1016/j.cis.2010.12.002)
Y. S. R. Elnaggar, Int. J. Nanomedicine 10 (2015) 3955 (https://doi.org/10.2147/IJN.S82558)
M. Stojancevic, N. Pavlovic, S. Golocorbin-Kon, M. Mikov, Front. Life Sci. 7 (2013) 112 (https://doi.org/10.1080/21553769.2013.879925)
C. Faustino, C. Serafim, P. Rijo, C. Pinto Reis, Expert Opin. Drug Deliv. 13 (2016) 1133 (https://doi.org/10.1080/17425247.2016.1178233)
D. Madenci, S. U. Egelhaaf, Curr. Opin. Colloid Interface Sci. 15 (2010) 109 (https://doi.org/10.1016/j.cocis.2009.11.010)
D. M. Small, S. A. Penkett, D. Chapman, Biochim. Biophys. Acta 176 (1969) 178 (https://doi.org/10.1016/0005-2760(69)90086-1)
D. M. Small, Advances in Chemistry Series, E. D. Goddard, Ed., Plenum Press, New York, 1968, p. 31 (https://doi.org/10.1021/ba-1968-0084.ch004)
S. Gouin, X. X. Zhu, Langmuir 14 (1998) 4025 (https://doi.org/10.1021/la971155w)
M. Posa, A. Sebenji, Biochim. Biophys. Acta - Gen. Subj. 1840 (2014) 1072 (https://doi.org/10.1016/j.bbagen.2013.11.008)
M. Haustein, P. Schiller, M. Wahab, H.-J. Mögel, J. Sol. Chem. 43 (2014) 1755
(https://doi.org/10.1007/s10953-014-0239-3)
M. Posa, A. Pilipovic, J. Mol. Liq. 238 (2017) 48 (https://doi.org/10.1016/j.molliq.2017.04.109)
R. Thakur, A. Das, C. Adhikari, A. Chakraborty, Phys. Chem. Chem. Phys. 16 (2014) 15681 (https://doi.org/10.1039/C4CP01308E)
S. Mandal, S. Ghosh, H. H. K. Aggala, C. Banerjee, V. G. Rao, N. Sarkar, Langmuir 29 (2013) 133 (https://doi.org/10.1021/la304319r)
S. Mandal, S. Ghosh, C. Banerjee, V. G. Rao, N. Sarkar, J. Phys. Chem., B 116 (2012) 8780 (https://doi.org/10.1021/jp302435h)
M. Gomez-Mendoza, E. Nuin, I. Andreu, M. Luisa Marin, M. A. Miranda, J. Phys. Chem., B 116 (2012) 10213 (https://doi.org/10.1021/jp304708y)
H. Nakazawa, N. R. Bachur, F. E. Chou, M. M. Mossoba, P. L. Gutierrez, Biophys. Chem. 21 (1985) 137 (https://doi.org/10.1016/0301-4622(85)85015-8)
R. Bittman, L. Blau, Biochemistry 14 (1975) 2138 (https://doi.org/10.1021/bi00681a015)
H. K. S. Souza, Thermochim. Acta 501 (2010) 1 (https://doi.org/10.1016/j.tca.2009.12.012)
H. E. Auer, FEBS Lett. 73 (1977) 167 (https://doi.org/10.1016/0014-5793(77)80973-3)
H. A. Benesi, J. H. Hildebrand, J. Am. Chem. Soc. 71 (1949) 2703 (https://doi.org/10.1021/ja01176a030)
L. Sepulveda, E. Lissi, F. Quina, Adv. Colloid Int. Sci. 25 (1986) 1 (https://doi.org/10.1016/0001-8686(86)80001-X)
H. Kawamura, M. Manabe, Y. Miyamoto, Y. Fujita, S. Tokunaga, J. Phys. Chem. 93 (1989) 5536 (https://doi.org/10.1021/j100351a042)
R. Li, E. Carpentier, E. D. Newell, L. M. Olague, E. Heafey, C. Yihwa, C. Bohne, Langmuir 25 (2009) 13800 (https://doi.org/10.1021/la901826y)
L. B. Partay, M. Sega. P. Jedlovszky, Langmuir 23 (2007) 12322 (https://doi.org/10.1021/la701749u)
G. B. Ray, I. Chakraborty, S. P. Moulik, J. Colloid Interface Sci. 294 (2006) 248 (https://doi.org/10.1016/j.jcis.2005.07.006)
L. Stopkova, J. Galisinova, Z. Suchtova, J. Cizmarik, F. Andriamainty, Molecules 23 (2018) (https://doi.org/10.3390/molecules23051064)
B. Natalini, R. Sardella, A. Gioiello, F. Ianni, A. Di Michele, M. Marinozzi, J. Pharm. Biomed. Anal. 87 (2014) 62 (https://doi.org/10.1016/j.jpba.2013.06.029)
T. S. Wiedmann, L. Kamel, J. Pharm. Sci. 91 (2002) 1743 (https://doi.org/10.1002/jps.10158)
K. Kumar, B. S. Patial, S. Chauhan, J. Chem. Thermodyn. 82 (2015) 25 (http://dx.doi.org/10.1016/j.jct.2014.10.014)
А. Mukherjee, S. Ghosh, M. Pal, B. Singh, J. Mol. Liq. 289 (2019) 111116 (https://doi.org/10.1016/j.molliq.2019.111116)
F. Westerlund, M. Wilhelmsson, B. Norden, P. Lincoln, J. Am. Chem. Soc. 125 (2003) 3773 (https://doi.org/10.1021/ja029243c)
А. Patra, S. Hazra, G. S. Kumar, R. K. Mitra, J. Phys. Chem., B 118 (2014) 901 (https://doi.org/10.1021/jp4091816)
M. Enache, S. Ionescu, E. Volanschi, J. Mol. Liq. 208 (2015) 333 (https://doi.org/10.1016/j.molliq.2015.05.006)
А. M. Toader, I. Dascalu, M. Enache, Acta Chim. Slov. 69 (2022) 331 (https://dx.doi.org/10.17344/acsi.2021.7189)
А. Das, C. Adhikari, D. Nayak, A. Chakraborty, Langmuir 32 (2016) 159 (https://doi.org/10.1021/acs.langmuir.5b03702)
А. Das, C. Adhikari, A. Chakraborty, Langmuir 32 (2016) 8889 (https://doi.org/10.1021/acs.langmuir.6b01860)
N. Maurya, Z. A. Parray, J. K. Maurya, A. Islam, R. Patel, ACS Omega 4 (2019) 21005 (https://doi.org/10.1021/acsomega.9b02246).