Chemically-assisted DNA transfection methods – An overview Review
Main Article Content
Abstract
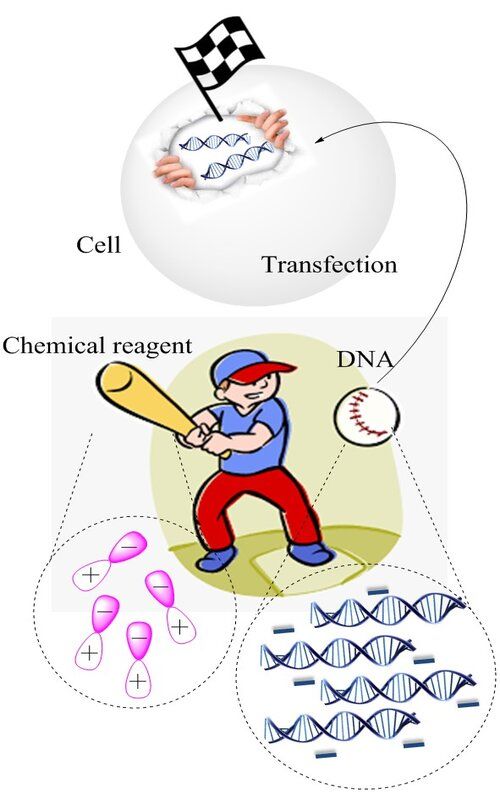
Non-viral chemical-based methods for in vitro cell transfection are commonly used to incorporate foreign gene of interest into mammalian cells due to numerous benefits – high efficiency, low cost and simple methodology. These powerful transfection methods generally do not possess safety risks as virus-based, and cell toxicity is significantly reduced. To obtain transfectants, host cells are usually treated with biocompatible DNA carriers such as calcium phosphate, cationic lipids, DEAE-dextran, polyethylenimine or dendrimers, classifying these methods based on chemical reagents used. All these different approaches are based on the similar principle, namely formation of encapsulated amphiphilic complexes between DNA and various particles, following cell uptake, most likely mediated by endocytosis. Depending on the aim and design of experiment, the choice of appropriate method is made. This review article outlines strategies of the most widely used chemical transfection techniques, pointing out advantages and limitations of different DNA carriers, also findings of researchers as how to optimize and enhance efficiency of gene delivery procedure. With methodology constantly being improved, transfection methods described here find their main, biomedical application in gene therapy, a promising way to introduce functional copy of exogenous gene to genetically defective target cells.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200125
References
C. Hardee, L. Arévalo-Soliz, B. Hornstein, L. Zechiedrich, Genes (Basel) 8 (2017) 65 (https://doi.org/10.3390/genes8020065)
J. Valsalakumari, J. Baby, E. Bijin, I. Constantine, S. Manjila, K. Pramod, Int. J. Pharm. Investig. 3 (2013) 1 (https://doi.org/10.4103/2230-973X.108958)
R. Zhou, R. C. Geiger, D. A. Dean, Expert Opin. Drug Deliv. 1 (2004) 127 (https://doi.org/10.1517/17425247.1.1.127)
B. Neuhaus, B. Tosun, O. Rotan, A. Frede, A. M. Westendorf, M. Epple, RSC Adv. 6 (2016) 18102 (https://doi.org/10.1039/c5ra25333k)
I. González-Domínguez, N. Grimaldi, L. Cervera, N. Ventosa, F. Gòdia, Nat. Biotechnol. 49 (2019) 88 (https://doi.org/10.1016/j.nbt.2018.09.005)
W. T. Godbey, K. K. Wu, A. G. Mikos, J. Control. Release 60 (1999) 149 (https://doi.org/10.1016/s0168-3659(99)00090-5)
F. M. Wurm, Nat. Biotechnol. 22 (2004) 1393 (https://doi.org/10.1038/nbt1026)
E. Wells, A. S. Robinson, Biotechnol. J. 12 (2017) 1600105 (https://doi.org/10.1002/biot.201600105)
W. A. Keown, C. R. Campbell, R. S. Kucherlapati, in Methods Enzymol., 1990, pp. 527–
–537 (https://doi.org/10.1016/0076-6879(90)85043-n)
A. Fus-Kujawa, P. Prus, K. Bajdak-Rusinek, P. Teper, K. Gawron, A. Kowalczuk, A. L. Sieron, Front. Bioeng. Biotechnol. 9 (2021) 701031 (https://doi.org/10.3389/fbioe.2021.701031)
J.A. Lee, A. Cho, E. N. Huang, Y. Xu, H. Quach, J. Hu, A. P. Wong, J. Transl. Med. 19 (2021) 452 (https://doi.org/10.1186/s12967-021-03099-4)
A. C. Nathwani, Hematology 2022 (2022) 569 (https://doi.org/10.1182/hematology.2022000388)
J. S. Chamberlain, Hum. Mol. Gen. 11 (2002) 2355 (https://doi.org/10.1093/hmg/11.20.2355)
D. Cross, J. K. Burmester, Clin Med Res. 4 (2006) 218 (https://doi.org/10.3121/cmr.4.3.218)
N. Sayed, P. Allawadhi, A. Khurana, V. Singh, U. Navik, S. K. Pasumarthi, I. Khurana, A. K. Banothu, R. Weiskirchen, K. K. Bharani, Life Sci. 294 (2022) 120375 (https://doi.org/10.1016/j.lfs.2022.120375)
T. I. Cornu, C. Mussolino, M. C. Müller, C. Wehr, W. V. Kern, T. Cathomen, Hum. Gene Ther. 32 (2021) 52 (https://doi.org/10.1089/hum.2020.159)
E. Hanna, C. Rémuzat, P. Auquier, M. Toumi, J. Mark. Access Heal. Policy 5 (2017) 1265293 (https://doi.org/10.1080/20016689.2017.1265293)
M. M. Lufino, P. A. Edser, R. Wade-Martins, Mol. Ther. 16 (2008) 1525 (https://doi.org/10.1038/mt.2008.156)
T. K. Kim, J. H. Eberwine, Anal. Bioanal. Chem. 397 (2010) 3173 (https://doi.org/10.1007/s00216-010-3821-6)
R. E. Kingston, C. A. Chen, H. Okayama, Curr. Protoc. Mol. Biol. 14 (1991) 9.1.1 (https://doi.org/10.1002/j.1934-3647.1991.tb00206.x)
G. S. Pari, Y. Xu, in Gene Deliv. to Mamm. Cells, Humana Press, Totowa, NJ, pp. 25–32 (https://doi.org/10.1385/1-59259-649-5:25)
A. A. Stepanenko, H. H. Heng, Mutat. Res. 773 (2017) 91 (https://doi.org/10.1016/j.mrrev.2017.05.002)
S. Patil, Y. Gao, X. Lin, Y. Li, K. Dang, Y. Tian, W. Zhang, S. Jiang, A. Qadir, A.-R. Qian, Int. J. Mol. Sci. 20 (2019) 5491 (https://doi.org/10.3390/ijms20215491)
S. Chenuet, D. Martinet, N. Besuchet-Schmutz, M. Wicht, N. Jaccard, A. Bon, M. Derouazi, D. L. Hacker, J. S. Beckmann, F. M. Wurm, Biotechnol. Bioeng. 101 (2008) 937 (https://doi.org/10.1002/bit.21972)
F. L. Graham, A. J. Van der Eb, Virology 52 (1973) 456 (https://doi.org/10.1016/0042-6822(73)90341-3)
P. Batard, M. Jordan, F. Wurm, Gene 270 (2001) 61 (https://doi.org/10.1016/S0378-1119(01)00467-X)
K. Khosravi-Darani, M. R. Mozafari, L. Rashidi, M. Mohammadi, Acta Med. Iran. 48 (2010) 133 (https://pubmed.ncbi.nlm.nih.gov/21137647)
T. Welzel, I. Radtke, W. Meyer-Zaika, R. Heumann, M. Epple, J. Mater. Chem. 14 (2004) 2213 (https://doi.org/10.1039/b401644k)
D. Olton, J. Close, C. Sfeir, P. N. Kumta, Biomaterials 32 (2011) 7662 (https://doi.org/10.1016/j.biomaterials.2011.01.043)
G. Ling, W. Liyang, Y. Ronghua, F. Rui, L. Zhongguang, M. Nishi, Z. Qi, W. Isaacs, J. Ma, X. Xuehong, Saudi J. Biol. Sci. 24 (2017) 622 (https://doi.org/10.1016/j.sjbs.2017.01.034)
C. Chen, H. Okayama, Mol. Cell. Biol. 7 (1987) 2745 (https://doi.org/10.1128/mcb.7.8.2745-2752.1987)
M. Jordan, A. Schallhorn, F. M. Wurm, Nucleic Acids Res. 24 (1996) 596 (https://doi.org/10.1093/nar/24.4.596)
M. Sun, L. P. Bernard, V. L. Dibona, Q. Wu, H. Zhang, J. Vis. Exp. 81 (2013) 1 (https://doi.org/10.3791/50808)
I. K. Sariyer, Methods Mol Biol. 1078 (20163) 133 (https://doi.org/10.1007/978-1-62703-640-5)
J. L. Huang, H. Chen, X. L. Gao, J. Drug Target. 26 (2018) 398 (https://doi.org/10.1080/1061186X.2017.1419360)
T. Liu, A. Tang, G. Zhang, Y. Chen, J. Zhang, S. Peng, Z. Cai, Cancer Biother. Radiopharm. 20 (2005) 141 (https://doi.org/10.1089/cbr.2005.20.141)
B. Ma, S. Zhang, H. Jiang, B. Zhao, H. Lv, J. Control Release 123 (2007) 184 (https://doi.org/10.1016/j.jconrel.2007.08.022)
T. W. R. Lee, D. A. Matthews, G. E. Blair, Biochem. J. 387 (2005) 1 (https://doi.org/10.1042/BJ20041923)
P. L. Felgner, T. R. Gadek, M. Holm, R. Roman, H. W. Chan, M. Wenz, J. P. Northrop, G. M. Ringold, M. Danielsen, Proc. Natl. Acad. Sci. 84 (1987) 7413 (https://doi.org/10.1073/pnas.84.21.7413)
D. Niculescu-Duvaz, J. Heyes, C. J. Springer, Curr. Med. Chem. 10 (2003) 1233 (https://doi.org/10.2174/0929867033457476)
G. Byk, C. Dubertret, V. Escriou, M. Frederic, G. Jaslin, R. Rangara, B. Pitard, J. Crouzet, P. Wils, B. Schwartz, D. Scherman, J. Med. Chem. 41 (1998) 224 (https://doi.org/10.1021/jm9704964)
D. Zhi, Y. Bai, J. Yang, S. Cui, Y. Zhao, H. Chen, S. Zhang, Adv. Colloid Interface Sci. 253 (2018) 117 (https://doi.org/10.1016/j.cis.2017.12.006)
P. Shende, N. Ture, R. S. Gaud, F. Trotta, Int. J. Pharm. 558 (2019) 250 (https://doi.org/10.1016/j.ijpharm.2018.12.085)
Y. Xu, F. C. Szoka, Biochemistry 35 (1996) 5616 (https://doi.org/10.1021/bi9602019)
F. Recillas-Targa, Mol. Biotechnol. 34 (2006) 337 (https://doi.org/10.1385/MB:34:3:337)
K. Romøren, B. J. Thu, N. C. Bols, Ø. Evensen, Biochim. Biophys. Acta 1663 (2004) 127 (https://doi.org/10.1016/j.bbamem.2004.02.007)
C. Charcosset, A. Juban, J. Valour, S. Urbaniak, H. Fessi, Chem. Eng. Res. Des. (2014) 1 (https://doi.org/10.1016/j.cherd.2014.09.008)
A. Zidovska, H. M. Evans, A. Ahmad, K. K. Ewert, C. R. Safinya, J. Phys. Chem., B 113 (2009) 5208 (https://doi.org/10.1021/jp809000e)
J. Yang, L. Huang, Gene Ther. 4 (1997) 950 (https://doi.org/10.1038/sj.gt.3300485)
P. C. Ross, S. W. Hui, Gene Ther. 6 (1999) 651 (https://doi.org/10.1038/sj.gt.3300863)
Z. Du, M. M. Munye, A. D. Tagalakis, M. D. I. Manunta, S. L. Hart, Sci. Rep. 4 (2014) 1 (https://doi.org/10.1038/srep07107)
R. K. Oskuee, M. Ramezanpour, L. Gholami, B. Malaekeh-Nikouei, Brazilian J. Pharm. Sci. 53 (2017) 1 (https://doi.org/10.1080/17458080.2013.771245)
S. W. Hui, M. Langner, Y. Zhao, P. Ross, E. Hurley, K. Chan, Biophys. J. 71 (1996) 590 (https://doi.org/10.1016/S0006-3495(96)79309-8)
D. Afonso, T. L. Gall, H. Couthon-Gourves, A. Grelard, S. Prakash, M. Berchel, N. Kervarec, E. J. Dufourc, Soft Matter 12 (2016) 4516 (https://doi.org/10.1039/c6sm00609d)
E. S. Hosseini, M. Nikkhah, S. Hosseinkhani, Int. J. Nanomedicine 14 (2019) 4353 (https://doi.org/10.2147/IJN.S199104)
J. Zhang, Q. Li, Y. Wu, D. Wang, L. Xu, Y. Zhang, S. Wang, T. Wang, F. Liu, M. Y. Zaky, S. Hou, S. Liu, K. Zou, H. Lei, L. Zou, Y. Zhang, H. Liu, Cell Commun. Signal. 17 (2019) 15 (https://doi.org/10.1186/s12964-019-0328-4)
M. C. Filion, N. C. Phillips, Br. J. Pharmacol. 122 (1997) 551 (https://doi.org/10.1038/sj.bjp.0701396)
D. Christensen, K. S. Korsholm, P. Andersen, E. M. Agger, Expert Rev. Vaccines 10 (2011) 513 (https://doi.org/10.1586/erv.11.17)
C. Lonez, M. F. Lensink, M. Vandenbranden, J.M. Ruysschaert, Biochim. Biophys. Acta 1790 (2009) 425 (https://doi.org/10.1016/j.bbagen.2009.02.015)
R. Rai, S. Alwani, I. Badea, Polymers (Basel) 11 (2019) 745 (https://doi.org/10.3390/polym11040745)
K. M. Luly, H. Yang, S. J. Lee, W. Wang, S. D. Ludwig, H. E. Tarbox, D. R. Wilson, J. J. Green, J. B. Spangler, Int. J. Nanomed. 17 (2022) 4469 (https://doi.org/10.2147/IJN.S377371)
S. Barua, J. Ramos, T. Potta, D. Taylor, H.-C. Huang, G. Montanez, K. Rege, Comb. Chem. High Throughput Screen. 14 (2011) 908 (https://doi.org/10.2174/138620711797537076)
S. T. Smale, Cold Spring Harb Protoc 2 (2010) 1 (https://doi.org/10.1101/pdb.prot5373)
H. Lv, S. Zhang, B. Wang, S. Cui, J. Yan, J. Control. Release 114 (2006) 100 (https://doi.org/10.1016/j.jconrel.2006.04.014)
M. E. Davis, Curr. Opin. Biotechnol. 13 (2002) 128 (https://doi.org/10.1016/s0958-1669(02)00294-x)
S. Pustylnikov, D. Sagar, P. Jain, Z. K. Khan, J. Pharm. Pharm. Sci. 17 (2014) 371 (https://doi.org/10.18433/J3N590)
S. J. Hong, M. H. Ahn, J. Sangshetti, P. H. Choung, R. B. Arote, Carbohydr. Polym. 181 (2018) 1180 (https://doi.org/10.1016/j.carbpol.2017.11.105)
P. A. Longo, J. M. Kavran, M. Kim, D. J. Leahy, Methods Enzym. (2013) 227 (https://doi.org/10.1016/B978-0-12-418687-3.00018-5)
W. G. Liu, K. De Yao, J. Control. Release 83 (2002) 1 (https://doi.org/10.1016/s0168-3659(02)00144-x)
A. Vaheri, J. S. Pagano, Virol 27 (1965) 434 (https://doi.org/10.1016/0042-6822(65)90126-1)
C. I. Cámara, N. Wilke, Chem. Phys. Lipids 204 (2017) 34 (https://doi.org/10.1016/j.chemphyslip.2017.03.005)
H. Luthman, G. Magnusson, Nucleic Acids Res. 11 (1983) 1295 (https://doi.org/10.1093/nar/11.5.1295)
T. Takai, H. Ohmori, Biochim. Biophys. Acta 1048 (1990) 105 (https://doi.org/10.1016/0167-4781(90)90029-2)
C. Wu, Y. Lu, Cell Mol. Biol. 53 (2010) 67 (www.ncbi.nlm.nih.gov/pmc/articles/PMC2830788)
P. Menon, T. Y. Yin, M. Misran, Colloids Surfaces, A 481 (2015) 345 (https://doi.org/10.1016/j.colsurfa.2015.05.036)
M. A. Lopata, D. W. Cleveland, B. Sollner-Webb, Nucleic Acids Res. 12 (1984) 5707 (https://doi.org/10.1093/nar/12.14.5707)
J. Sussman, G. Milman, Mol. Cell. Biol. 4 (1984) 1641 (https://doi.org/10.1128/mcb.4.8.1641-1643.1984)
K. D. Mack, R. Wei, A. Elbagarri, N. Abbey, M. S. McGrath, J. Immunol. Methods 211 (1998) 79 (https://doi.org/10.1016/s0022-1759(97)00194-4)
Y. Onishi, Y. Eshita, A. Murashita, M. Mizuno, J. Yoshida, Nanomedicine 3 (2007) 184 (https://doi.org/10.1016/j.nano.2007.07.002)
S. Patnaik, A. Aggarwal, S. Nimesh, A. Goel, M. Ganguli, N. Saini, Y. Singh, K. C. Gupta, J. Control. Release 114 (2006) 398 (https://doi.org/10.1016/j.jconrel.2006.06.025)
O. Boussif, F. Lezoualch, M. A. Zanta, M. D. Mergny, D. Schermant, B. Demeneixt, J. P. Behr, Proc. Natl. Acad. Sci. USA 92 (1995) 7297 (https://doi.org/10.1073/pnas.92.16.7297)
M. X. Tang, F. C. Szoka, Gene Ther. 4 (1997) 823 (https://doi.org/10.1038/sj.gt.3300454)
S. Gutiérrez-Granados, L. Cervera, A. A. Kamen, F. Gòdia, Crit. Rev. Biotechnol. 38 (2018) 918 (https://doi.org/10.1080/07388551.2017.1419459)
Z. Rezvani Amin, M. Rahimizadeh, H. Eshghi, A. Dehshahri, M. Ramezani, Iran. J. Basic Med. Sci. 16 (2013) 150 (https://doi.org/10.22038/ijbms.2013.295)
Y. H. Choi, F. Liu, J.-S. Kim, Y. K. Choi, Jong Sang Park, S. W. Kim, J. Control. Release 54 (1998) 39 (https://doi.org/10.1016/S0168-3659(97)00174-0)
G. Storm, S. O. Belliot, T. Daemen, D. D. Lasic, Adv. Drug Deliv. Rev. 17 (1995) 31 (https://doi.org/10.1016/0169-409X(95)00039-A)
D. D. Dunlap, A. Maggi, M. R. Soria, L. Monaco, Nucleic Acids Res. 25 (1997) 3095 (https://doi.org/10.1093/nar/25.15.3095)
D. L. Hacker, D. Kiseljak, Y. Rajendra, S. Thurnheer, L. Baldi, F. M. Wurm, Protein Expr. Purif. 92 (2013) 67 (https://doi.org/10.1016/j.pep.2013.09.001)
V. Viswanath, K. Santhakumar, J. Photochem. Photobiol., B 173 (2017) 61 (https://doi.org/10.1016/j.jphotobiol.2017.05.023)
D. G. Shcharbin, B. Klajnert, M. Bryszewska, Biochem. 74 (2009) 1070 (https://doi.org/10.1134/s0006297909100022)
J. F. Kukowska-Latallo, A. U. Bielinska, J. Johnson, R. Spindler, D. A. Tomalia, J. R. Baker, Proc. Natl. Acad. Sci. USA 93 (1996) 4897 (https://doi.org/10.1073/pnas.93.10.4897)
E. Pedziwiatr-Werbicka, K. Milowska, V. Dzmitruk, M. Ionov, D. Shcharbin, M. Bryszewska, Eur. Polym. J. 119 (2019) 61 (https://doi.org/10.1016/j.eurpolymj.2019.07.013)
F. S. T. Mirakabad, M. S. Khoramgah, K. Keshavarz F., M. Tabarzad, J. Ranjbari, Life Sci. 233 (2019) 1 (https://doi.org/10.1016/j.lfs.2019.116754)
F. Xie, R. Li, W. Shu, L. Zhao, J. Wan, Mater. Today Biol. 14 (2022) 100239 (https://doi.org/10.1016/j.mtbio.2022.100239)
M. Wang, Y. Cheng, Biomaterials 35 (2014) 6603 (https://doi.org/10.1016/j.biomaterials.2014.04.065)
M. X. Tang, C. T. Redemann, F. C. Szoka, Bioconjug. Chem. 7 (1996) 703 (https://doi.org/10.1021/bc9600630)
A. Kwok, G. A. Eggimann, J. Reymond, T. Darbre, F. Hollfelder, ASC Nano 7 (2013) 4668 (https://doi.org/10.1021/nn400343z).