Copper leaching from the chalcopyrite-bearing MoS2 concentrate by mixed chlorides solution Scientific paper
Main Article Content
Abstract
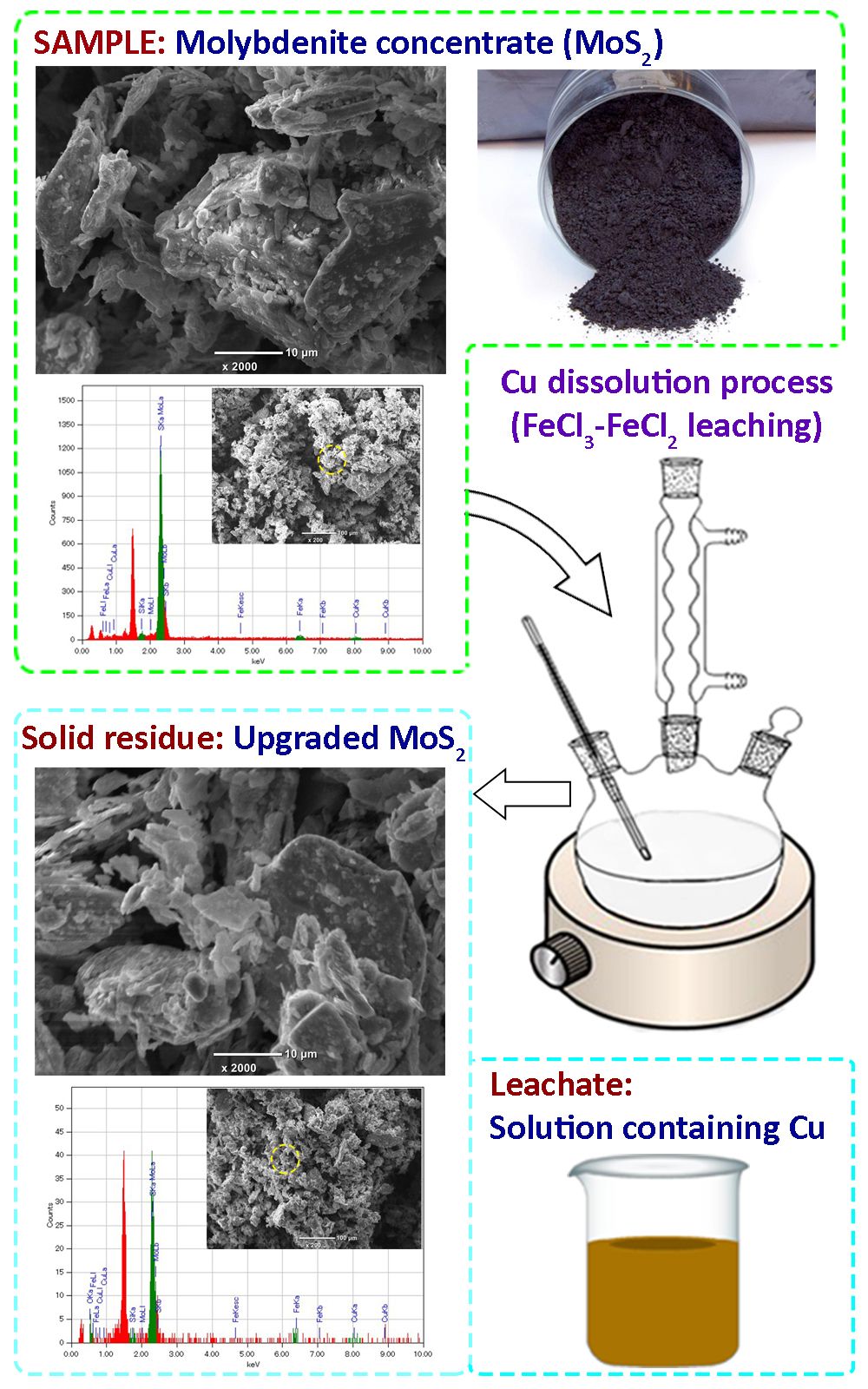
In this study, the dissolution of copper sulfide minerals by the ferric (FeCl3) and ferrous (FeCl2) chloride leaching for upgrading the content of molybdenum disulfide (MoS2) in a molybdenite concentrate was investigated. The effect of various parameters was studied on the copper dissolution behaviour from the concentrate. In this matter, the copper dissolution was reached 94.84 % under the optimized leaching conditions. The kinetics of copper dissolution from the concentrate was established using a shrinking core model (SCM), and the process was controlled by diffusion, with a corresponding activation energy of 18.63 kJ mol-1 at the temperature range of 343–373 K. The amount of copper in the leachate was tested by the inductively coupled plasma-optical emission spectrometer (ICP-OES) and the solid phase was studied by X-ray diffraction (XRD), and scanning electron microscope (SEM). Results of the experiments show that the content of MoS2 in the solid residue was increased up to 88.59 % after the leaching.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Mongolian Foundation for Science and Technology
Grant numbers (2021-2023)
References
C. K. Gupta, Chemical Metallurgy, Principles and Practice, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2003 (ISBN: 3-527-30376-6)
A. R. Lansdown, Molybdenum Disulfide Lubrication, Elsevier, Swansea, 1999 (ISBN: 0-444-50032-4)
O. Samy, A. El Moutaouakil, Energies 14 (2021) 1 (https://doi.org/10.3390/en14154586)
D. Gupta, V. Chauhan, R. Kumar, Inorg. Chem. Commun. 121 (2020) 108200 (https://doi.org/10.1016/j.inoche.2020.108200)
E. L. R. Chiluiza, P. N. Donoso, J. Mex. Chem. Soc. 60 (2016) 238 (ISSN: 1870-249X)
N. Tumen-Ulzii, A. Batnasan, B. Gunchin, Miner. Eng. 185 (2022) 107715 (https://doi.org/10.1016/j.mineng.2022.107715)
R. Padilla, C. Opazo, M. C. Ruiz, Metall. Mater. Trans., B 46 (2015) 30 (https://doi.org/10.1007/s11663-014-0171-3)
R. Padilla, H. Letelier, M. C. Ruiz, Hydrometallurgy 137 (2013) 78 (https://doi.org/10.1016/j.hydromet.2013.05.012)
H. L. Jennings, P.H., Stanley, R.W. Ames, in Proceedings of Second Int. Symp. Hydrometall. New York (AIME), 1973, pp. 868–883
D. Guo, L. Fu, S. Wang, L. Zhang, J. Peng, Chem. Pap. 72 (2018) 2997 (https://doi.org/10.1007/s11696-018-0544-1)
A. A. Baba, K. I. Ayinla, F. A. Adekola, M. K. Ghosh, O. S. Ayanda, R. B. Bale, A. R. Sheik, S. R. Pradhan, Int. J. Min. Eng. Miner. Process. 1 (2012) 1 (https://doi.org/10.5923/j.mining.20120101.01)
M. Nicol, H. Miki, L. Velásquez-Yévenes, Hydrometallurgy 103 (2010) 86 (https://doi.org/10.1016/j.hydromet.2010.03.003)
Y. Li, F. Wang, B. Yang, J. Wu, & Y. Tian, J. Sustain. Metall. 6 (2020) 419 (https://doi.org/10.1007/s40831-020-00284-5)
N. T. Phuong Thao, S. Tsuji, S. Jeon, I. Park, C. B. Tabelin, M. Ito, N. Hiroyoshi, Hydrometallurgy 194 (2020) 105299 (https://doi.org/10.1016/j.hydromet.2020.105299)
N. Hiroyoshi, H. Miki, T. Hirajima, M. Tsunekawa, Hydrometallurgy 60 (2001) 185 (https://doi.org/10.1016/S0304-386X(00)00155-9)
J. Lu, D. Dreisinger, Miner. Eng. 45 (2013) 185 (https://doi.org/10.1016/j.mineng.2013.03.007)
O. Levenspiel, Electrochemical Reaction Engineering, John Willey & Sons, New York, 1999, ISBN: 0-471-25424-X
T. Agacayak, M. T. O. A. Ahmed, Acta Chem. Iasi 28 (2020) 82 (https://doi.org/10.2478/achi-2020-0006)
E. M. Córdoba, J. A. Muñoz, M. L. Blázquez, F. González, A. Ballester, Hydrometallurgy 93 (2008) 81 (https://doi.org/10.1016/j.hydromet.2008.04.015)
Y. Turkmen, E. Kaya, J. Ore Dress. 11 (2009) 16
M. E. Taboada, P. C. Hernández, A. P. Padilla, N. E. Jamett, T. A. Graber, Metals 11 (2021) 866 (https://doi.org/10.3390/met11060866)
T. Hidalgo, L. Kuhar, A. Beinlich, A. Putnis, Hydrometallurgy 188 (2019) 140 (https://doi.org/10.1016/j.hydromet.2019.06.009)
T. Havlík, Hydrometallurgy, Principles and Application, CRC Press, Boca Raton, FL, 2008 (ISBN: 978-1-84569-461-6).