Click mediated synthesis of functionalized glycolipids with peptide-peptoid linkages Scientific paper
Main Article Content
Abstract
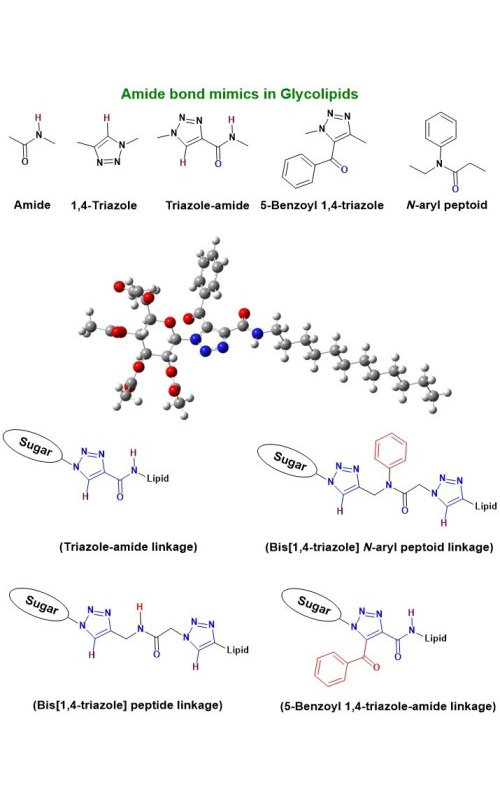
The present work describes the synthesis of a new class of glycolipids with systematic variations in the linkage region, as well as in the aglycon part using Cu(I) catalyzed click reaction. The linkage region between sugar and the aglycon part was diversified using amide, amido-triazole and 5-benzoyl triazole moieties. The structural diversity of glycolipids was further amplified by incorporating several polar peptide foldamer groups such as triazole, amide, peptide, or N-aryl peptoid in the aglycon part. The newly designed glycolipids were derived from the amalgamation of different peptide bond mimics. This work reports the first use of N-aryl peptoid in the synthesis of glycolipids. The newly synthesized glycolipids were characterized using different spectroscopic and spectrometric analyses. The impact of the amide bond as well as the triazole ring in the linkage region on the morphology of the glycolipids was analysed by comparing their self-assemblies using SEM analysis. The geometries of the glycolipids were also optimized using density functional theory and the optimized structures were found to be minima in the potential energy surfaces.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Science and Engineering Research Board
Grant numbers TAR/2018/000764
References
R. C. R. Jala., S. Vudhgiri., C. G. Kumar, Carbhydr. Res. 516 (2022) 108556 (https://doi.org/10.1016/j.carres.2022.108556)
Y. Queneau, S. Chambert, C. Besset, R. Cheaib, Carbohydr. Res. 343 (2008) 1999 (https://doi.org/10.1016/j.carres.2008.02.008)
-L. Chaveriat, I. Gosselin, C. Machut, P. Martin, Eur. J. Med. Chem. 62 (2013) 177 (https://doi.org/10.1016/j.ejmech.2012.12.032)
D. Wu, M. Fujio, C.-H. Wong, Bioorg. Med. Chem. 16 (2008) 1073 (https://doi.org/10.1016/j.bmc.2007.10.026)
T. Lee, M. Cho, S.-Y. Ko, H.-J. Youn, D. J. Baek, W.-J. Cho, C.-Y. Kang, S. Kim, J. Med. Chem. 50 (2007) 585 (https://doi.org/10.1021/jm061243q)
R. A. Falconer, I. Toth, Bioorg. Med. Chem. 15 (2007) 7012 (https://doi.org/10.1016/j.bmc.2007.07.048)
L. Sahoo, A. Singhamahapatra, S. Sahoo, J. Serb. Chem. Soc. 83 (2018) 539 (https://doi.org/10.2298/JSC170905030S)
H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. Int. Ed. 40 (2001) 2004 (https://doi.org/10.1002/1521-773(20010601)40:11<2004::AIDANIE2004>3.0.CO;2-5)
J. E. Moses, A. D. Moorhouse, Chem. Soc. Rev. 36 (2007) 1249 (https://doi.org/10.1039/B613014N)
V. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh, X. Chen, Chem. Rev. 116 (2016) 3086 (https://doi.org/10.1021/acs.chemrev.5b00408)
A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra, V. K. Tiwari, Chem. Rev. 121 (2021) 7638 (https://doi.org/10.1021/acs.chemrev.0c00920)
K. J. V. Paul, D. Loganathan, Tetrahedron Lett. 49 (2008) 6356 (https://doi.org/10.1016/j.tetlet.2008.08.073)
H.-L. Zhang, X.-P. He, L. Sheng, Y. Yao, W. Zhang, X.-X. Shi, J. Li, G.-R. Chen, Mol. Divers 15 (2011) 889 (https://doi.org/10.1007/s11030-011-9318-1)
V. Neto, R. Granet, P. Krausz, Tetrahedron 66 (2010) 4633 (https://doi.org/10.1016/j.tet.2010.03.115)
A. Baron, Y. Blériot, M. Sollogoub, B. Vauzeilles, Org. Biomol. Chem. 6 (2008) 1898 (https://doi.org/10.1039/B805528A)
F. A. Sani, T. Heidelberg, R. Hashim, Farhanullah, Colloids Surf. B: Biointerfaces 97 (2012) 196 (https://doi.org/10.1016/j.colsurfb.2012.03.030)
S.-X. Song, H.-L. Zhang, C.-G. Kim, L. Sheng, X.-P. He, Y.-T. Long, J. Li, G.-R. Chen, Tetrahedron 66 (2010) 9974 (https://doi.org/10.1016/j.tet.2010.10.033)
M. J. Clemente, J. Fitremann, M. Mauzac, J. L. Serrano, L. Oriol, Langmuir 27 (2011) 15236 (https://doi.org/10.1021/la203447e)
K. Pérez-Labrada, I. Brouard, I. Méndez, C. S. Pérez, J. A. Gavin, D. G. Rivera, Eur. J. Org Chem. (2014) 3671 (https://doi.org/10.1002/ejoc.201402117)
A. Singhamahapatra, L. Sahoo, D. Loganathan, J. Org. Chem. 78 (2013) 10329 (https://doi.org/10.1021/jo401720s)
L. Sahoo, A. Singhamahapatra, D. Loganathan, Org. Biomol. Chem. 12 (2014) 2615 (https://doi.org/10.1039/C3OB42308E)
L. Sahoo, S. Kundu, A. Singhamahapatra, N. K. Jena, G. C. Nayak, S. Sahoo, Carbohydr. Res. 469 (2018) 23 (https://doi.org/10.1016/j.carres.2018.08.015)
S. M. Miller, R. J. Simon, S. Ng, R. N. Zuckermann, J. M. Kerr, W. H. Moos, Drug Dev. Res. 35 (1995) 20 (https://doi.org/10.1002/ddr.430350105)
J. T. Nguyen, C. W. Turck, F. E. Cohen, R. N. Zuckermann, W. A. Lim, Science 282 (1998) 2088 (https://doi.org/10.1126/science.282.5396.2088)
N. H. Shah, G. L. Butterfoss, K. Nguyen, B. Yoo, R. Bonneau, D. L. Rabenstein, K. Kirshenbaum, J. Am. Chem. Soc. 130 (2008) 16622 (https://doi.org/10.1021/ja804580n)
K. J. V. Paul, L. Sahoo, V. Sorna, D. Loganathan, Trends Carbohydr. Res. 2 (2010) 21 (https://www.trendscarbo.com/getf_shoppingcart.php?id=726840555)