Comparative study of chemical composition and the antimutagenic activity of propolis extracts obtained by means of various solvents Scientific paper
Main Article Content
Abstract
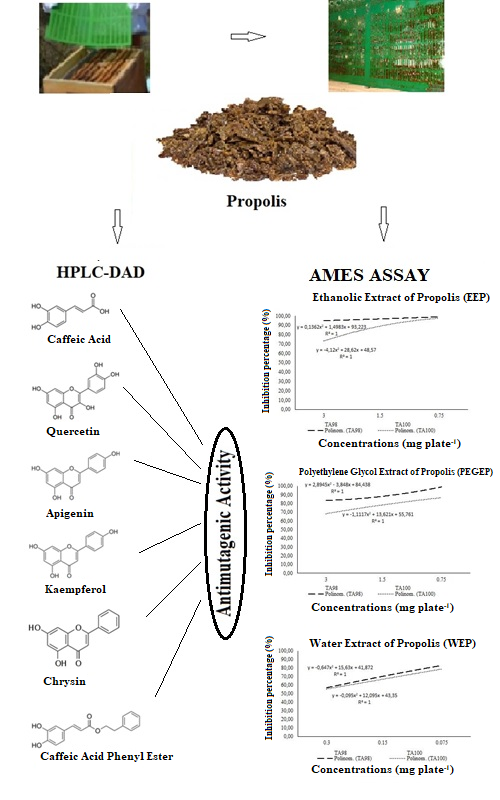
The present study is aimed to evaluate the chemical characterization and antimutagenic potential of propolis extracted in three different solvents (ethanol, polyethylene glycol and water). The chemical properties of different extracts of propolis were identified using HPLC-DAD and LC–MS/MS and polyethylene glycol extract of propolis were found to be richer than the ethanolic and water extracts of propolis considering chemical composition. In addition, the antimutagenic activities of propolis extracts were determined using Ames assay. The concentrations of 3, 1.5 and 0.75 mg plate-1 of ethanolic and polyethylene glycol extracts, as well as 0.3, 0.15 и 0.075 mg plate-1 of water extract of propolis were used as active materials. Propolis extracted in three different solvents indicated strong antimutagenic activity against both 4-nitro-
-o-phenylendiamine and sodium azide mutagens in the Salmonella typhimurium TA98 and 100 strains at all concentrations. Ethanolic extract of propolis had the highest inhibition rates for both bacterial strains and these rates were 98.94 and 97.37 % for TA98 and TA100, respectively. The inhibition rates of polyethylene glycol extract of propolis ranged from 68.27 to 98.94%. Moreover, it was determined that water extract of propolis had the lowest inhibition rates, which were 56.86 and 55.35% for TA98 and TA100, respectively. The toxicological safety of natural products such as propolis has gained great importance due to extensive usage.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
I. Przybyłek, T. M. Karpinski, Mol. 24 (2019) 2047 (https://doi.org/10.3390/molecules24112047)
D. Kasote, V. Bankova, A. M. Viljoen, Phytochem. Rev. 1 (2022) 25 (https://doi.org/10.1007/s11101-022-09816-1)
M. Jug, M. Z. Koncic, I. Kosalec, LWT Food Sci. Technol. 57 (2014) 530 (https://doi.org/10.1016/j.lwt.2014.02.006)
R. D. Vargas-Sánchez, A. M. Mendoza-Wilson, G. R. Torrescano-Urrutia, A. Sánchez-Escalante, Comput. Theor. Chem. 1066 (2015) 7 (https://doi.org/10.1016/j.comptc.2015.05.003)
A. F. N. Ramos, J. L. Miranda, J. Venom Anim. Toxins Incl. Trop. Dis. 13 (2007) 697 (https://doi.org/10.1590/S1678-91992007000400002)
J. M. Sforcin, Phytother. Res. 30 (2016) 894 (https://doi.org/10.1002/ptr.5605)
M. L. F. Bittencourt, P. R. Ribeiro, R. L.P. Franco, H. W. M. Hilhorst, R. D. de Castro, L. G. Fernandez, Food Res. Int. 76 (2015) 449 (https://doi.org/10.1016/j.foodres.2015.07.008)
J. M. Sforcin, V. Bankova, J. Ethnopharmacol. 133 (2011) 253 (https://doi.org/10.1016/j.jep.2010.10.032)
F. I. Abdullaev, L. Riveron-Negrete, H. Caballero-Orgeta, J. M. Hernandez, I. Perez-Lopez, R. Pereda-Miranda, J. J. Espinosa-Aguirre, Toxicol. In Vitro 17 (2003) 731 (https://doi.org/10.1016/s0887-2333(03)00098-5)
E. H. Çakır, Y. Şirin, S. Kolaylı, Z. Can, J. Apither. Nat. 1 (2018) 13 (https://dergipark.org.tr/en/download/article-file/449611)
B. J. Dean, T. M. Brooks, G. Hodsonwalker, D. H. Hutson, Mutat. Res. 153 (1985) 57 (https://doi.org/10.1016/0165-1110(85)90005-3)
K. Mortelmans, E. Zeiger, Mutat. Res. 455 (2000) 29 (https:/doi.org/10.1016/s0027-5107(00)00064-6)
D. M. Maron, B. N. Ames, Mutat. Res. 113 (1983) 173 (https://doi.org/10.1016/0165-1161(83)90010-9)
P. S. Negi, G. K. Jayaprakasha, B. S. Jena, Food Chem. 80 (2003) 393 (https://doi.org/10.1016/S0308-8146(02)00279-0)
I. Sofrenić, J. Ljujić, K. Simić, S. Ivanović, J. S. Jeremić, A. Ćirić, M. Soković, B. Anđelković, J. Serb. Chem. Soc. 86 (2021) 1205 (https://doi.org/10.2298/JSC210812086S)
M. Arslan, Y. Sevgiler, C. Guven, Z. T. Murathan, N. Erbil, D. Yildirim, M. Buyukleyla, S. Karadas, R. Celik, E. Rencuzogullari, Arh. Hig. Rada. Toksikol. 72 (2021) 53 (https://doi.org/10.2478/aiht-2021-72-3492)
A. Sorucu, H. H. Oruc, J. Food Measur. Character. 13 (2019) 2461 (https://doi.org/10.1007/s11694-019-00166-9)
E. Sevim, A. Bozdeveci, M. Pinarbaş, M. Kekeçoğlu, R. Akpinar, M. Keskin, S. Kolayli, S. A. Karaoğlu, U. Bee J. 21 (2021) 177 (https://doi.org/10.31467/uluaricilik.976536)
M. Donmez, S. Karadeniz, T. Yoldas, G. Aydin, P. Karagul, O. Aksu, P. G. Rasgele, IJTCMR 1 (2020) 137 (https://dergipark.org.tr/en/download/article-file/1407649)
T. Ozdal, F. D. Ceylan, N. Eroglu, M. Kaplan, E. O. Olgun, E. Capanoglu, Food Res. Internat. 122 (2019) 528 (https://doi.org/10.1016/j.foodres.2019.05.028)
P. G. Rasgele, M. Kekecoglu, J. Apither. Nat. 1 (2018) 20 (https://dergipark.org.tr/en/download/article-file/562828)
R. Chang, D. Piló-Veloso, S. A. L. Morais, E. A. Nascimento, Brazilian J. Pharmacogn. 18 (2008) 549 (https://doi.org/10.1590/S0102-695X2008000400009)
F. G. da Silva Dantas, P. F. de Castilho, A. A. de Almeida-Apolonio, R. P. de Araújo, K. M. P. de Oliveira, Mutat. Res./Reviews Mutat. Res. 786 (2020) 108338 (https://doi.org/10.1016/j.mrrev.2020.108338)
K. Słoczyńska, B. Powroźnik, E. Pękala, A. M. Waszkielewicz, J. Appl. Genetics 55 (2014) 273 (https://doi.org/10.1007/s13353-014-0198-9)
M. M. Nichitoi, A. M. Josceanu, R. D. Isopescu, G. O. Isopencu, E. I. Geana, C. T. Ciucure, V. Lavric, Sci. Rep. 11 (2021) 1 (https://doi.org/10.1038/s41598-021-97130-9)
D. López-Romero, J. A. Izquierdo-Vega, J. A. Morales-González, E. Madrigal-Bujaidar, G. Chamorro-Cevallos, M. Sánchez-Gutiérrez, G. Betanzos-Cabrera, I. Alvarez-Gonzalez, A. Morales-González, E. Madrigal-Santillán, Nutrients 10 (2018) 1954 (https://doi.org/10.3390/nu10121954)
M. Majidinia, A. Bishayee, B. Yousefi, DNA Repair 82 (2019) 102679 (https://doi.org/10.1016/j.dnarep.2019.102679)
E. A. Varanda, R. Monti, D. C. Tavares, Teratogen. Carcinogen. Mutagen. 19 (1999) 403 ( https://doi.org/10.1002/(SICI)1520-6866(1999)19:6<403::AID-TCM4>3.0.CO;2-2)
S. N. Jeng, M. K. Shih, C. M. Kao, T. Z. Liu, S. C. Chen, Food Chem. Toxicol. 38 (2000) 893 (https://doi.org/10.1016/s0278-6915(00)00081-8)
J. Y. Fu, Y. Xia, Y. Y. Zheng, Biomed. Environ. Sci. 17 (2004) 469 (https://www.besjournal.com/en/article/id/ef7d9720-e640-4de1-b0aa-8829172e2920)
M. I. N. Moreno, I. C. Zampini, R. M. Ordóñez, G. S. Jaime, M. A. Vattuone, M. I. Isla, J. Agric. Food Chem. 53 (2005) 8957 (https://doi.org/10.1021/jf0513359)
N. E. Bayram, M. Karadayi, M. Gulluce, S. Bayram, K. Sorkun, G. C. Oz, M. N. Aydogan, T. Y. Koc, B. Alaylar, B. Salih, Mellifera 15 (2015) 29 (https://dergipark.org.tr/en/pub/mellifera/issue/17834/186991)
R. O. A. Lima, A. P. Bazo, R. A. Said, J. M. Sforcin, V. Bankova, B. R. Darros, D. M. F. Salvadori, Environ. Mol. Mutagen. 45 (2005) 8 (https://doi.org/10.1002/em.20082)
M. M. Roberto, S. T. Matsumoto, C. M. Jamal, O. Malaspina, M. A. Marin-Morales, Toxicol. In Vitro 33 (2016) 9 (https://doi.org/10.1016/j.tiv.2016.02.005).