Thermoanalytical and spectroscopic studies on medicated jellies with perphenazine Scientific paper
Main Article Content
Abstract
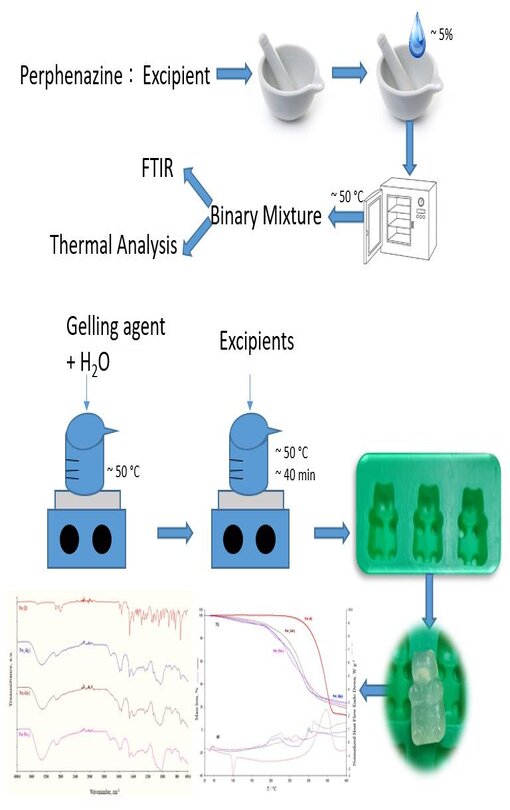
Medicated jellies are widely used by a large part of patients, especially by people with swallowing difficulties. Preformulation studies play an essential role in the development of new pharmaceutical formulations. The present study aimed to formulate and evaluate medicated jellies containing perphenazine, an antipsychotic drug from the group of phenothiazine compounds used to treat schizophrenia and other mental disorders. Typical gelling agents such as sodium alginate (Alg), gelatine (Gel), and pectin (Pec) were used to develop the medicated jellies. In addition to the biopolymers, components such as benzoic acid (BenzAc), citric acid (CitAc), sodium citrate (NaCit), sorbitol (Sorb) and xylitol (Xyl) were also used. Before preparing the jellies, the moist binary mixture between each component of the jelly and the active substance was analysed to investigate the compatibility of the substances. The active substance, moist binary mixture, and medicated jellies were analysed by FTIR_UATR spectroscopy, UV–Vis spectroscopy and thermogravimetry.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. Sarojini, K. Anusha, C. Maneesha, M. A. Mufaquam, B. Deepika, Y. Krishna Reddy, N. R. Kandukoori, World J. Pharm. Res. 7 (2014) 352 (https://doi.org/10.20959/wjpr20186-11502)
K. H. Kim, M. Jun, M. K. Lee, Pharmaceutics 12 (2020) 1 (https://doi.org/10.3390/pharmaceutics12111073)
A. D. Darade, A. S. Mundada, World J. Pharm. Res. 10 (2021) 1628 (https://doi.org/10.20959/wjpr20216-20658)
L. E. Committee, USP – Nomenclature Guidelines, 2006 (https://doi.org/10.1038/sj.bjp.0706759)
S. Sunil, U. K. Sharma, S. A. Arathy, J. Pharm. Sci. Res. 12 (2020) 904
A. Lutka, W. Prange, Acta Pol. Pharm. 62 (2005) 419
Z. Ramezani, M. Shekarriz, A. A. Behfar, S. Kiamarzi, J. Braz. Chem. Soc. 28 (2017) 2172 (https://doi.org/10.21577/0103-5053.20170066)
S. Hong, M. yeong Lee, K. S. Shin, S. J. Kang, Animal Cells Syst. (Seoul). 16 (2012) 20 (https://doi.org/10.1080/19768354.2011.611256)
S. Beg, S. Swain, M. Rizwan, M. Irfanuddin, D. Shobha Malini, Curr. Drug Deliv. 8 (2011) 691 (https://doi.org/10.2174/156720111797635504)
S. Baboota, G. Mustafa, J. Sahni, J. Ali, J. Excipients Food Chem. 4 (2013) 12 (https://jefc.scholasticahq.com/article/1099-mechanistic-approach-for-the-development-of-ultrafine-oil-water-emulsions-using-monoglyceride-and-blends-of-medium-and-long-chain-triglycerides-enhan)
Z. Saghafi, M. Mohammadi, M. M. Mahboobian, K. Derakhshandeh, Drug Dev. Ind. Pharm. 47 (2021) 509 (https://doi.org/10.1080/03639045.2021.1892745)
L. Wang, F. Zeng, L. Zong, Pharm. Dev. Technol. 18 (2013) 1101 (https://doi.org/10.3109/10837450.2012.700932)
S. H. Almurisi, A. A. Doolaanea, M. E. Akkawi, B. Chatterjee, K. Ahmed Saeed Aljapairai, M. Z. Islam Sarker, Drug Dev. Ind. Pharm. 46 (2020) 1373 (https://doi.org/10.1080/03639045.2020.1791165)
Z. H. Mahdi, Al Mustansiriyah J. Pharm. Sci. 20 (2020) (https://doi.org/10.32947/ajps.v20i3.765)
E. Gomaa, M. M. Ayoub, Saudi Pharm. J. 29 (2021) 955 (https://doi.org/10.1016/j.jsps.2021.07.020)
S. Patel, N. Scott, K. Patel, V. Mohylyuk, W. J. McAuley, F. Liu, J. Pharm. Sci. 109 (2020) 2474 (https://doi.org/10.1016/j.xphs.2020.04.018)
A. Hassen Elshafeey, R. Moataz El-Dahmy, Saudi Pharm. J. 30 (2022) 1435 (https://doi.org/10.1016/j.jsps.2022.07.004)
D. Okolišan, G. Vlase, T. Vlase, C. Avram, Polymers (Basel) 14 (2022) (https://doi.org/10.3390/polym14204275)
N. K. Sachan, P. Seema, A. Jha, A. Bhattcharya, J. Pharm. Res. 2 (2009) 1191
V. S. Kadam, J. Kendre, G. R. Shendarkar, S. S. Kadam, Int. J. Pharm. Sci. Res. 11 (2020) 6251 (https://doi.org/10.13040/IJPSR.0975-8232.11(12).6251-59)
M. Chamoli, S. K. Tangri, Int. J. Sci. Res. 11 (2022) 1541 (https://doi.org/10.21275/SR22521115404)
K. Prakash, V. M. Satyanarayana, H. T. Nagiat, A. H. Fathi, A. K. Shanta, A. R. Prameela, Asian J. Pharm. 8 (2014) 241 (https://doi.org/10.4103/0973-8398.143937)
M. Budiul, C. A. Marioane, I. A. Bradu, G. Vlase, T. Vlase, J. Therm. Anal. Calorim. 148 (2023) 4589 (https://doi.org/10.1007/s10973-022-11882-8)
M. Mateescu, G. Vlase, M. M. Budiul, B. D. Cernuşcă, T. Vlase, J. Therm. Anal. Calorim. 148 (2023) 4601 (https://doi.org/10.1007/s10973-023-12052-0)
S. H. Kumar, A. Sheikalisha, S. Chandra, R. Suresh, S. Sangeetha, Int. J. Adv. Pharm. Sci. 1 (2018) 7
R. Taranum, S. Mittapally, J. Drug Deliv. Ther. 8 (2018) 65 (https://doi.org/10.22270/jddt.v8i4.1784)
K. V. S, J. Kendre, G. R. Shendarkar, S. S. Kadam, Int. J. Pharm. Sci. Res. 11 (2020) 6251 (https://doi.org/10.13040/IJPSR.0975-8232.11(12).6251-59)
A. Jaszczyszyn, K. Ga̧siorowski, P. Świa̧tek, W. Malinka, K. Cieślik-Boczula, J. Petrus, B. Czarnik-Matusewicz, Pharmacol. Rep. 64 (2012) 16 (https://doi.org/10.1016/S1734-1140(12)70726-0)
S. H. Almurisi, K. Al-Japairai, F. Alshammari, F. Alheibshy, R. M. F. Sammour, A. A. Doolaanea, Gels 8 (2022) 1 (https://doi.org/10.3390/gels8030144).