Synthesis, antimycobacterial and antifungal evaluation of new 4-(furan-2-ylmethyl)-6-methylpyridazin-3(2H)-ones Scientific paper
Main Article Content
Abstract
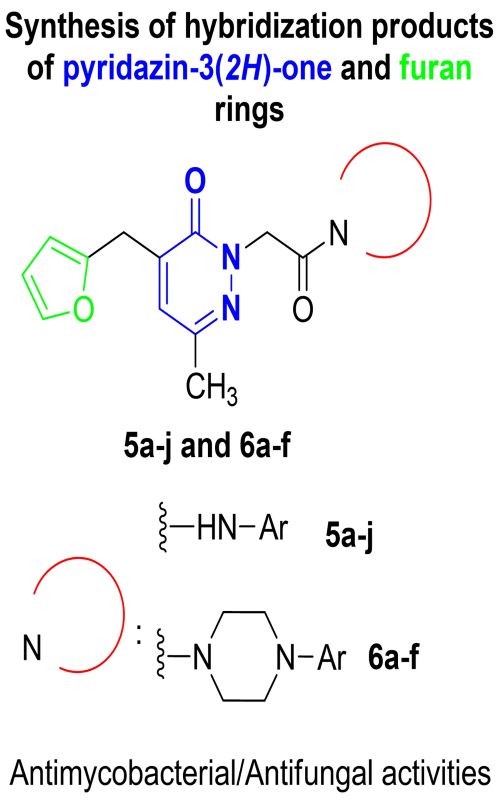
This study reports the synthesis and evaluation of a series of new pyridazin-3-ones with furan moieties 5a–j and 6a–f, to test for their antimycobacterial and antifungal activities. The structures of the target compounds were confirmed by elemental analysis and spectroscopic techniques (IR, mass, 1H- and 13C-NMR). Amongst the compounds tested, 5e, 5g, 5i and 6e exhibited highest activity against Mycobacterium tuberculosis, while 5h, 6d and 6f showed moderate in vitro antifungal activities against Candida albicans and Candida parapsilosis.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Norges Forskningsråd
Grant numbers 261669;309592 -
Joint Programming Initiative on Antimicrobial Resistance
Grant numbers 298410
References
World Health Organization, Global Tuberculosis Report, 2022
M. M. Sirim, V. S. Krishna, D. Sriram, O. Unsal Tan, Eur. J. Med. Chem. 188 (2020) 112010 (https://doi.org/10.1016/j.ejmech.2019.112010)
D. Falzon, H. J. Schünemann, E. Harausz, L. González-Angulo, C. Lienhardt, E. Jaramillo, K. Weyer, Eur. Respir. J. 49 (2017) 1 (http://dx.doi.org/10.1183/13993003.02308-2016)
A. Koch, H. Cox, V. Mizrahi, Curr. Opin. Pharmacol. 42 (2018) 7 (https://doi.org/10.1016/j.coph.2018.05.013)
V. Dartois, Nat. Rev. Microbiol. 12 (2014) 159 (https://doi.org/10.1038/nrmicro3200)
H. Getahun, A. Matteelli, R. E. Chaisson, M. Raviglione, N. Eng. J. Med. 372 (2015) 2127 (https://doi.org/10.1056/NEJMRA1405427)
G. Grüber, Prog. Biophys. Mol. Biol. 152 (2020) 2 (https://doi.org/10.1016/J.PBIOMOLBIO.2020.02.003)
O. U. Tan, K. Ozadali, P. Yogeeswari, D. Sriram, A. Balkan, Med. Chem. Res. 21 (2012) 2388 (http://dx.doi.org/10.1007/s00044-011-9770-6)
D. Mantu, M. C. Luca, C. Moldoveanu, G, Zbancioc, I. I. Mangalagiu, Eur. J. Med. Chem. 45 (2010) 5164 (http://dx.doi.org/10.1016/J.EJMECH.2010.08.029)
H. B. V. Sowmya, T. S. Kumara, G. Nagendrappa, J. P. Jasinski, S. P. Millikan, G. Jose, Dileep R., P. S. Sujan Ganapathy, J. Mol. Struct. 1054 (2013) 179 (http://dx.doi.org/10.1016/J.MOLSTRUC.2013.09.046)
G. Zhou, P. C. Ting, R. Aslanian, J. Cao, D. W. Kim, R. Kuang, J. F. Lee, J. Schwerdt, H. Wu, R. J. Herr, A. J. Zych, J. Yang, S. Lam, S. Wainhaus, T. A. Black, P. M. McNicholas, Y. Xu, S. S. Walker, Bioorg. Med. Chem. Lett. 21 (2011); 2890 (http://dx.doi.org/10.1016/J.BMCL.2011.03.083)
D. Li, P. Zhan, H. Liu, C. Pannecouque, J. Balzarini, E. De Clercq, X. Liu, Bioorg. Med. Chem. 21 (2013); 2134 (http://dx.doi.org/10.1016/J.BMC.2012.12.049)
J. Singh, D. Sharma, R. Bansal, Future Med. Chem. 9 (2016) 95 (http://dx.doi.org/10.4155/FMC-2016-0194)
X. Zhang, J. Luo, Q, Li, Q. Xin, L. Ye, Q. Zhu, Z. Shi, F. Zhan, B. Chu, Z. Liu, Y. Jiang, Eur. J. Med. Chem. 226 (2021) 113812 (http://dx.doi.org/10.1016/J.EJMECH.2021.113812)
A. A. Siddiqui, S. Partap, S. Khisal, M. Shahar Yar, R. Mishra, Bioorg. Chem. 99 (2020) 103584 (http://dx.doi.org/10.1016/J.BIOORG.2020.103584)
S. Utku, M. Gökçe, G. Aslan, G. Bayram, Turkish J. Chem. 35 (2011); 331 (http://dx.doi.org/10.3906/kim-1009-63)
M. Asif, M. Imran, Anal. Chem. Lett. 10 (2020) 414 (http://dx.doi.org/10.1080/22297928.2020.1776633)
M. Asif, A. Singh, S. A. Khan, A. Husain, Brazilian J. Pharm. Sci. 54 (2018); 1 (http://dx.doi.org/10.1590/S2175-97902018000300040)
M. Asif, A. Imran, M. Imran, IJPSR 11 (2020) 826 (http://dx.doi.org/ 10.13040/IJPSR.0975-8232.11(2).826-31)
L. R. Chiarelli, M. Mori, G. Beretta, A. Gelain, E. Pini, J. C. Sammartino, G, Stelitano, D. Barlocco, L. Costantino, M. Lapillo, G, Poli, I. Caligiuri, F, Rizzolio, M. Bellinzoni, T. Tuccinardi, S. Villa, F. Meneghetti, J. Enzyme Inhib. Med. Chem. 34 (2019) 823 (http://dx.doi.org/10.1080/14756366.2019.1589462)
L. R. Chiarelli, M. Mori, D. Barlocco, G. Beretta, A. Gelain, E. Pini, M. Porcino, G. Mori, G. Stelitano, L. Costantino, M. Lapillo, D. Bonanni, G. Poli, T. Tuccinardi, S. Villa, F. Meneghett, Eur. J. Med. Chem. 155 (2018) 754 (http://dx.doi.org/10.1016/j.ejmech.2018.06.033)
R. P. Tangallapally, R. Yendapally, R. E. Lee, K. Hevener. V. C. Jones, A. J. M. Lenaerts, M. R. McNeil, Y. Wang, S. Franzblau, R. E. Lee, J. Med. Chem. 47 (2004) 5276 (http://dx.doi.org/10.1021/jm049972y)
Y. L. Fan, J. B. Wu, X. Ke, Z. P. Huang, Bioorg. Med. Chem. Lett. 28 (2018) 3064 (https://doi.org/10.1016/j.bmcl.2018.07.046)
R. P. Tangallapally, D. Sun, N. Budha, R. E. Lee, A. J. M. Lenaerts, N. Budha, R. E. Lee, Bioorg. Med. Chem. Lett. 17 (2007) 6638 (http://dx.doi.org/10.1016/j.bmcl.2007.09.048)
N. R. Tawari, R. Bairwa, M. K. Ray, M. G. R. Rajan, M. S. Degani, Bioorg. Med. Chem. Lett., 20 (2010); 6175 (http://dx.doi.org/10.1016/j.bmcl.2010.08.127)
N. H. Zuma, J. Aucamp, D. D. N’Da, Eur. J. Pharm. Sci. 140 (2019) 105092 (https://doi.org/10.1016/j.ejps.2019.105092)
N. H. Zuma, F. J. Smit, R. Seldon, J. Aucamp, A. Jordaan, D. F. Warner, D. D. N’Da, Bioorg. Chem. 96 (2020) 103587 (https://doi.org/10.1016/j.bioorg.2020.103587)
Z. Yang, Y. Sun, Q. Liu, A. Li, W. Wang, W. Gu, J. Agric. Food Chem. 69 (2021) 13373 (https://doi.org/10.1021/acs.jafc.1c03857)
A. Evidente, G. Cristinzio, B. Punzo, A. Andolfi, A. Testa, D. Melck, Chem. Biodivers. 6 (2009) 328 (http://dx.doi.org/10.1002/CBDV.200800292)
O. S. Trefzger, N. V. Barbosa, R. L. Scapolatempo, A. R. das Neves, M. L. F. S. Ortale, D. B. Carvalho, A. M. Honorato, M. R. Fragoso, C. Y. K. Shuiguemoto, R. T. Perdomo, M. F. C. Matos, M. R. Chang, C. C. P. Arruda, A. C. M. Baroni, Arch. Pharm.353 (2020) 1900241 (http://dx.doi.org/10.1002/ARDP.201900241)
G. B. Wang, L. F. Wang, C. Z. Li, J. Sun, G. M. Zhou, D. C. Yang, Res. Chem. Intermed. 38 (2012) (https://doi.org/10.1007/s11164-011-0327-6)
Clinical and Laboratory Standards Institute (CLSI), Ref. Method Broth Dilution Antifung. Susceptibility Test. Yeasts. Approv. Stand., 2008 (https://clsi.org/media/1461/m27a3_sample.pdf).