Defluoridation using pinecone-based activated carbon: Adsorption isotherm, kinetics, regeneration and co-ions effect investigation Scientific paper
Main Article Content
Abstract
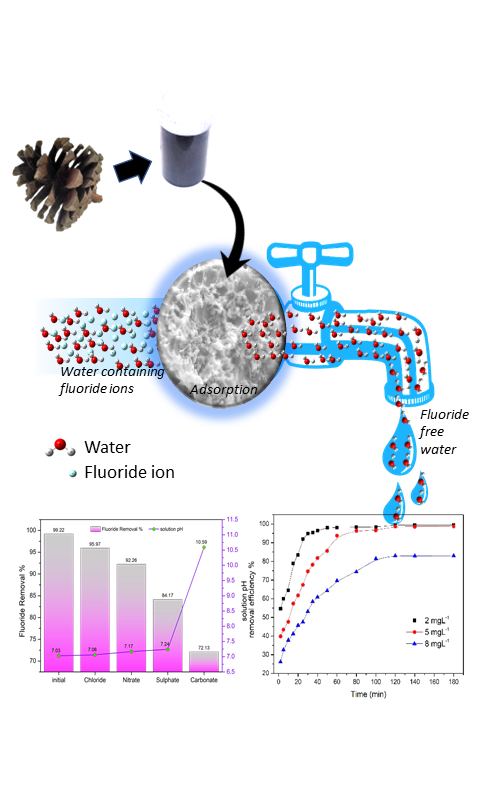
In this work, cheap and locally available pinecones of Pinus kiseya were used as a precursor to prepare activated carbon using single-step KOH activation for the removal of fluoride from water. The prepared activated carbon’s BET surface area, and total pore volume, were determined as 972.13 m2 g-1 and 0.469 cm3 g-1, respectively. Batch adsorption studies were evaluated at different contact times, solution pH, adsorbent dose and concentration to obtain the optimum conditions for maximum adsorption. The adsorption data were fitted with the the isotherm models (Langmuir, Freundlich ad Temkin isotherm model) and the adsorption kinetic models. The experimental data were found to best fit using the Langmuir isotherm which confirmed the formation of a monolayer coverage with a maximum adsorption capacity of 2.845 m2 g-1. The adsorption kinetics was well described by the pseudo-second-order model. A study on the effects of co-existing ions showed that fluoride adsorption capacity was observed to decrease in the order: CO32- > SO42- > NO3- > Cl-. The regeneration studies were investigated to determine the reusability of the spent adsorbent. In summary, these findings demonstrated substantial evidence that the activated carbon can be prepared from P. kiseya cones as an eco-friendly adsorbent for the removal of ions such as fluoride from water.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Department of Science and Technology, Ministry of Science and Technology, India
Grant numbers DST_INSPIRE FELLOWSHIP
References
WHO, Guidelines for drinking-water quality, Fourth ed., World Health Organization, Geneva, 2017
S. Jagtap, M. K. Yenkie, N. Labhsetwar, S. Rayalu, Chem. Rev. 112 (2012) 2454 (https://doi.org/10.1021/cr2002855)
R. L. Moirana, J. Mkunda, R. Machunda, M. Paradelo, K. Mtei, Environ. Adv. 11 (2023) 100329 (https://doi.org/10.1016/J.ENVADV.2022.100329)
P. C. Bhomick, A. Supong, R. Karmaker, M. Baruah, C. Pongener, D. Sinha, Korean J. Chem. Eng. 36 (2019) 551 (https://doi.org/10.1007/s11814-019-0234-x)
A. Bhatnagar, E. Kumar, M. Sillanpää, Chem. Eng. J. 171 (2011) 811 (https://doi.org/10.1016/j.cej.2011.05.028)
P. I. Ndiaye, P. Moulin, L. Dominguez, J. C. Millet, F. Charbit, Desalination 173 (2005) 25 (https://doi.org/10.1016/j.desal.2004.07.042)
S. Singh, A. Khare, S. Chaudhari, J. Environ. Chem. Eng. 8 (2020) 103704 (https://doi.org/10.1016/j.jece.2020.103704)
A. Amalraj, A. Pius, Appl. Water Sci. 7 (2017) 2653 (https://doi.org/10.1007/s13201-016-0479-z)
S. Bason, A. Ben-David, Y. Oren, V. Freger, Desalination 199 (2006) 31 (https://doi.org/10.1016/j.desal.2006.03.137)
Z. Amor, B. Bariou, N. Mameri, M. Taky, S. Nicolas, A. Elmidaoui, Desalination 133 (2001) 215 (https://doi.org/10.1016/S0011-9164(01)00102-3)
M. Grzegorzek, K. Majewska-Nowak, A. E. Ahmed, Sci. Total Environ. 722 (2020) 137681 (https://doi.org/10.1016/j.scitotenv.2020.137681)
R. C. Bansal, M. Goyal, Activated carbon adsorption, CRC Press, Boca Raton, FL, 2005
K. P. Gopinath, D. V. N. Vo, D. Gnana Prakash, A. Adithya Joseph, S. Viswanathan, J. Arun, Environ. Chem. Lett. 19 (2021) 557 (https://doi.org/10.1007/s10311-020-01084-9)
D. S. G. D. Senewirathna, S. Thuraisingam, S. Prabagar, J. Prabagar, Curr. Res. Green Sustain. Chem. 5 (2022) 100304 (https://doi.org/10.1016/J.CRGSC.2022.100304)
B. D. Gebrewold, P. Kijjanapanich, E. R. Rene, P. N. L. Lens, A. P. Annachhatre, Environ. Technol. (U.K.) 40 (2019) 2913 (https://doi.org/10.1080/09593330.2018.1459871)
J. Fito, H. Said, S. Feleke, A. Worku, Environ. Syst. Res. 8 (2019) 1 (https://doi.org/10.1186/s40068-019-0153-1)
D. E. Jayashree, P. S. Kumar, P. T. Ngueagni, D. V. N. Vo, K. W. Chew, Environ. Pollut. 272 (2021) 115969 (https://doi.org/10.1016/j.envpol.2020.115969)
C. Pongener, D. Kibami, K. S. Rao, R. L. Goswamee, D. Sinha, J. Water Chem. Technol. 39 (2017) 108 (https://doi.org/10.3103/S1063455X17020096)
C. Pongener, P. C. Bhomick, A. Supong, M. Baruah, U. B. Sinha, D. Sinha, J. Environ. Chem. Eng. 6 (2018) 2382 (https://doi.org/10.1016/j.jece.2018.02.045)
B. M. Babic, S. K. Milonjic, M. J. Polovina, B. V. Kaludierovic, Carbon N. Y. 37 (1999) 477 (https://doi.org/10.1016/S0008-6223(98)00216-4)
R. Araga, S. Soni, C. S. Sharma, J. Environ. Chem. Eng. 5 (2017) 5608 (https://doi.org/10.1016/j.jece.2017.10.023)
George Socrates, Infrared and Raman Characteristic Group Frequencies Contents, Third ed., Wiley, New York, 2001
L. M. Harwood, T. D. W. Claridge, Introduction to Organic Spectroscopy, Oxford University Press, 1997
B. Smith, Fundamentals of Fourier transform infrared spectroscopy, Second Ed., CRC Press, New York, 2011
A.-N. A. El-Hendawy, Appl. Surf. Sci. 255 (2009) 3723 (https://doi.org/10.1016/j.apsusc.2008.10.034)
O. Aksakal, H. Ucun, J. Hazard. Mater. 181 (2010) 666 (https://doi.org/10.1016/j.jhazmat.2010.05.064)
R. K. Gautam, P. K. Gautam, M. C. Chattopadhyaya, J. D. Pandey, Proc. Natl. Acad. Sci. India, A 84 (2014) 495 (https://doi.org/10.1007/s40010-014-0154-4)
S. Swarupa, J. Bersillon, K. Gopal, Separ. Purific. Technol. 50 (2006) 310 (https://doi.org/10.1016/j.seppur.2005.11.036)
M. H. Dehghani, M. Farhang, M. Alimohammadi, M. Afsharnia, G. Mckay, Chem. Eng. Commun. 205 (2018) 955 (https://doi.org/10.1080/00986445.2018.1423969)
R. Mallampati, S. Valiyaveettil, RSC Adv. 2 (2012) 9914 (https://doi.org/10.1039/c2ra21108d)
K. S. Lagergren, Sven. Vetenskapsakad. Handingarl 24 (1898) 1
G. Blanchard, M. Maunay, G. Martin, Water Res. 18 (1984) 1501 (https://doi.org/10.1016/0043-1354(84)90124-6)
Y. S. Ho, G. McKay, Process Biochem. 34 (1999) 451 (https://doi.org/10.1016/S0032-9592(98)00112-5)
W. J. Weber, J. C. Morris, J. Sanit. Eng. Div. 89 (1963) 31 (https://doi.org/10.1080/002689796173345)
D. Haddad, A. Mellah, D. Nibou, S. Khemaissia, J. Environ. Eng. (U.S.A.) 144 (2018) (https://doi.org/10.1061/(ASCE)EE.1943-7870.0001349)
R. Zaidi, S. U. Khan, I. H. Farooqi, A. Azam, RSC Adv. 11 (2021) 28744 (https://doi.org/10.1039/d1ra00598g)
S. K. Theydan, M. J. Ahmed, J. Anal. Appl. Pyrolysis 97 (2012) 116 (https://doi.org/10.1016/j.jaap.2012.05.008)
Z. Li, D. Xiao, Y. Ge, S. Koehler, ACS Appl. Mater. Interfaces 7 (2015) 15000 (https://doi.org/10.1021/acsami.5b03994)
S. P. Kumar, S. Ramalingam, C. Senthamarai, M. Niranjanaa, P. Vijayalakshmi, S. Sivanesan, Desalination 261 (2010) 52 (https://doi.org/10.1016/j.desal.2010.05.032)
J. Zhang, N. Chen, Z. Tang, Y. Yu, Q. Hu, C. Feng, Phys. Chem. Chem. Phys. 17 (2015) 12041 (https://doi.org/10.1039/C5CP00817D)
L. Xu, X. Gao, Z. Li, C. Gao, Desalination 369 (2015) 97 (https://doi.org/10.1016/j.desal.2015.04.033)
J. Saikia, S. Sarmah, T. H. Ahmed, P. J. Kalita, R. L. Goswamee, J. Environ. Chem. Eng. 5 (2017) 2488 (https://doi.org/10.1016/j.jece.2017.04.046)
M. S. Onyango, Y. Kojima, O. Aoyi, E. C. Bernardo, H. Matsuda, J. Colloid Interface Sci. 279 (2004) 341 (https://doi.org/10.1016/j.jcis.2004.06.038)
S. P. Kamble, S. Jagtap, N. K. Labhsetwar, D. Thakare, S. Godfrey, S. Devotta, S. S. Rayalu, Chem. Eng. J. 129 (2007) 173 (https://doi.org/10.1016/j.cej.2006.10.032)
Y. Chen, Q. Zhang, L. Chen, H. Bai, L. Li, J. Mater. Chem. A 1 (2013) 13101 (https://doi.org/10.1039/c3ta13285d).