A recent tactic for searching CDK-7 kinase inhibitor by NCI database screening Scientific paper
Main Article Content
Abstract
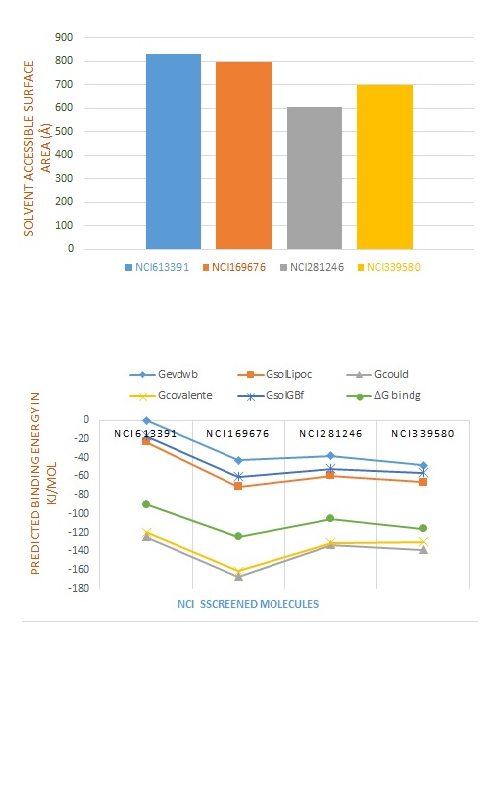
The present study was based on an exploration of NCI database for searching specific CDK-7 kinase inhibitor by HTVS, SP, XP, molecular docking, molecular dynamic simulation, and ADMET evaluation. The best CDK-7 kinase inhibitors (NCI613391, NCI169676, NCI281246, NCI339580) were identified via NCI database screening. The stability of binding interaction between receptor protein and protein-ligand complex of potent finding compounds (NCI613391) was further confirmed by dynamics simulations and MM-GBSA. The RMSD value of receptor and receptor–ligand complexes was analysed, and it revealed the stability of binding interactions and remained stable throughout the simulation. The RMSF values and gyration radius of the unbound receptor and backbone atoms of the complex were found to be equal, which indicates that the drug molecule inside the CDK7 receptor is also stable. The study of MM-GBSA data also revealed stronger binding interactions of ligands to CDK7 receptors. With the exception of NCI169676, all compounds were shown to be substrates for CYP450 2D6, CYP450 3A4, inhibitors of CYP450 2C9, and non-inhibitors of p-glycoprotein. All compounds were qualified and found suitable to be as drug-likeness according to the Lipinski rule, Ghose filter, MDDR like rule and CMC-like rule. The compound (NCI613391) exhibited human intestinal absorption (76.08%), displayed negative AMES and T.E.S.T (US-EPA) toxicity with OSIRIS property and found to be a promising CDK-7 kinase inhibitor and its efficacy may be further explored in clinical trials.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
L. Kolloch, T. Kreinest, M. Meisterernst, A. Oeckinghaus, Int. J. Mol. Sci. 23 (2022) 812 (http://dx.doi.org/10.3390/ijms23020812)
P. Hazel, S. H. Kroll, A. Bondke, M. Barbazanges, H. Patel, M. J. Fuchter, R. C. Coombes, S. Ali, A. G. Barrett, P. S. Freemont, Chem. Med. Chem. 13 (2018) 207 (http://dx.doi.org/10.1002/cmdc.201700826)
M. E. Noble, J. A. Endicott, L. N. Johnson, Science 303 (2004) 1800 (http://dx.doi.org/10.1126/science.1095920)
S. Larochelle, J. Chen, R. Knights, J. Pandur, P. Morcillo, H. Erdjument-Bromage, P. Tempst, B. Suter, R. P. Fisher, The EMBO J. 20 (2001) 3749 (http://dx.doi.org/10.1093/emboj/20.14.3749)
X. Li, D. C. Dean, J. Yuan, T. H. Temple, J. C. Trent, A. E. Rosenberg, S. Yu, F. J. Hornicek, Z. Duan, Biomed. Pharmacother. 149 (2022) 112888 (http://dx.doi.org/10.1016/j.biopha.2022.112888)
G. Lolli, L.N. Johnson, Cell Cycle 4 (2005) 565 (https://pubmed.ncbi.nlm.nih.gov/15876871/)
E. Chipumuro, E. Marco, C. L. Christensen, N. Kwiatkowski, T. Zhang, C. M. Hatheway, B. J. Abraham, B. Sharma, C. Yeung, A. Altabef, Cell 159 (2014) 1126 (http://dx.doi.org/10.1016/j.cell.2014.10.024)
Q. Li, S. Pan, T. Xie, H. Liu, Blood Sci. 3 (2021) 65 (http://dx.doi.org/10.1097/BS9.0000000000000073)
X. Barril, F. Javier Luque, J. Comput. Aided Mol. Des. 26 (2012) 81 (http://dx.doi.org/10.1007/s10822-011-9506-1)
F. Godschalk, S. Genheden, P. Söderhjelm, U. Ryde, Phys. Chem. Chem. Phys. 15 (2013) 7731 (http://dx.doi.org/10.1039/c3cp00116d)
G. Madhavi Sastry, M. Adzhigirey, T. Day, R. Annabhimoju, W. Sherman, J. Comput. Aided Mol. Des. 27 (2013) 221 (http://dx.doi.org/10.1007/s10822-013-9644-8)
Schrödinger Preparation Wizard, Lig prep. version 2.6, Glide version 5.8 (2012) (http://gohom.win/ManualHom/Schrodinger/Schrodinger_2012_docs/glide/glide_quick_start.pdf)
A. Hussain, C. K. Verma, Biomed. Res. 28 (2017) 5805 (https://www.alliedacademies.org/articles/molecular-docking-and-in-silico-admet-study-reveals-354aminomethyl-piperidin1yl-methyl1hindol2yl1hindazole6carbonitrile-as-a-poten.pdf)
A. Hussain, C. K. Verma, J. Cancer. Res. Ther. 15 (2019) 1131 (http://dx.doi.org/10.4103/jcrt.JCRT_47_18)
QikProp version 4.4 (2010), Schrödinger, LLC, New York, NY, 2015 (http://gohom.win/ManualHom/Schrodinger/Schrodinger_2015-2_docs/qikprop/qikprop_user_manual.pdf)
S. V. Pattar, S. A. Adhoni, C. M. Kamanavalli, S. S. Kumbar, Beni-Suef Univ. J. Basic Appl. Sci. 9 (2020) 36 (https://doi.org/10.1186/s43088-020-00059-7)
A. Hussain, C.K. Verma, U. Chouhan, Saudi J. Biol. Sci. 24 (2017) 1229 (http://dx.doi.org/10.1016/j.sjbs.2015.10.003)
V. Kumar, S. Parate, G. Thakur, G. Lee, H.-S. Ro, Y. Kim, H.J. Kim, M. O. Kim, K. W. Lee, Biomedicines 9 (2021) 1197 (http://dx.doi.org/10.3390/biomedicines9091197)
P. V. Rusina, I. Y. Titov, M. V. Panova, V. S. Stroylov, Y. R. Abdyusheva, E. Y. Murlatova, I. V. Svitanko, F. N. Novikov, Mendeleev Commun. 30 (2020) 430 (http://dx.doi.org/10.1016/j.mencom.2020.07.008)
C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug Deliv. Rev. 23 (1997) 3 (http://dx.doi.org/10.1016/s0169-409x(00)00129-0)
P. Ertl, B. Rohde, P. Selzer, J. Med. Chem. 43 (2000) 3714 (http://dx.doi.org/10.1021/jm000942e)
A. K. Ghose, V. N. Viswanadhan, J. J. Wendoloski, J. Comb. Chem. 1 (1999) 55 (http://dx.doi.org/10.1021/cc9800071)
T. I. Oprea, J. Comput. Aided Mol. Des. 14 (2000) 251 (http://dx.doi.org/10.1023/a:1008130001697)
A. Kulkarni, Y. Han, A. J. Hopfinger, J. Chem. Inf. Comput. Sci. 42 (2002) 331 (http://dx.doi.org/10.1021/ci010108d)
M. Rashid, Bioorg. Chem. 96 (2020) 103576 (http://dx.doi.org/10.1016/j.bioorg.2020.103576)
S. Kumar, F. Abbas, I. Ali, M. K. Gupta, S. Kumar, M. Garg, D. Kumar, Phytomed Plus 3 (2023) 100419 (https://doi.org/10.1016/j.phyplu.2023.100419)
M. Rashid, O. Afzal, A. S. A. Altamimi, J. Chil. Chem. Soc. 66 (2021) 5164 (http://dx.doi.org/10.4067/S0717-97072021000205164)
S. Thomas, Explorer, OSIRIS Property, Actelion Pharmaceuticals Ltd., Allschwil, 2001 (https://www.organic-chemistry.org/prog/peo/)
T. Martin, Toxicity estimation software tool (TEST), US Environmental Protection Agency, Washington DC, 2016 (https://www.epa.gov/sites/default/files/2016-05/documents/600r16058.pdf)
N. Sripriya, M. R. Kumar, N. A. Karthick, S. Bhuvaneswari, N. K. U. Prakash, Drug Chem. Toxicol. 44 (2019) 480 (http://dx.doi.org/10.1080/01480545.2019.1614023)
F. S. Prasetiya, W. Destiarani, I. R. C. Prihastaningtyas, M. U. K. Agung, M. Yusuf, J. Appl. Pharm. Sci. 13 (2023) 068 (http://dx.doi.org/10.7324/JAPS.2023.10012)
B. N. Ames, E. Gurney, J. A. Miller, H. Bartsch, Proc. Natl. Acad. Sci. USA 69 (1972) 3128 (http://dx.doi.org/10.1073/pnas.69.11.3128)
P. J. Eddershaw, M. Dickins, Pharm. Sci. Technol. Today 2 (1999) 13 (http://dx.doi.org/10.1016/s1461-5347(98)00108-4)
J. H. Lin, M. Yamazaki, Clin. Pharmacokinet. 42 (2003) 59 (http://dx.doi.org/10.2165/00003088-200342010-00003)
G. Lolli, E. D. Lowe, N. R. Brown, L. N. Johnson, Structure 12 (2004) 2067 (http://dx.doi.org/10.1016/j.str.2004.08.013).