A DFT study of the chemical bonding properties, aromaticity indexes and molecular docking study of some phenylureas herbicides Scientific paper
Main Article Content
Abstract
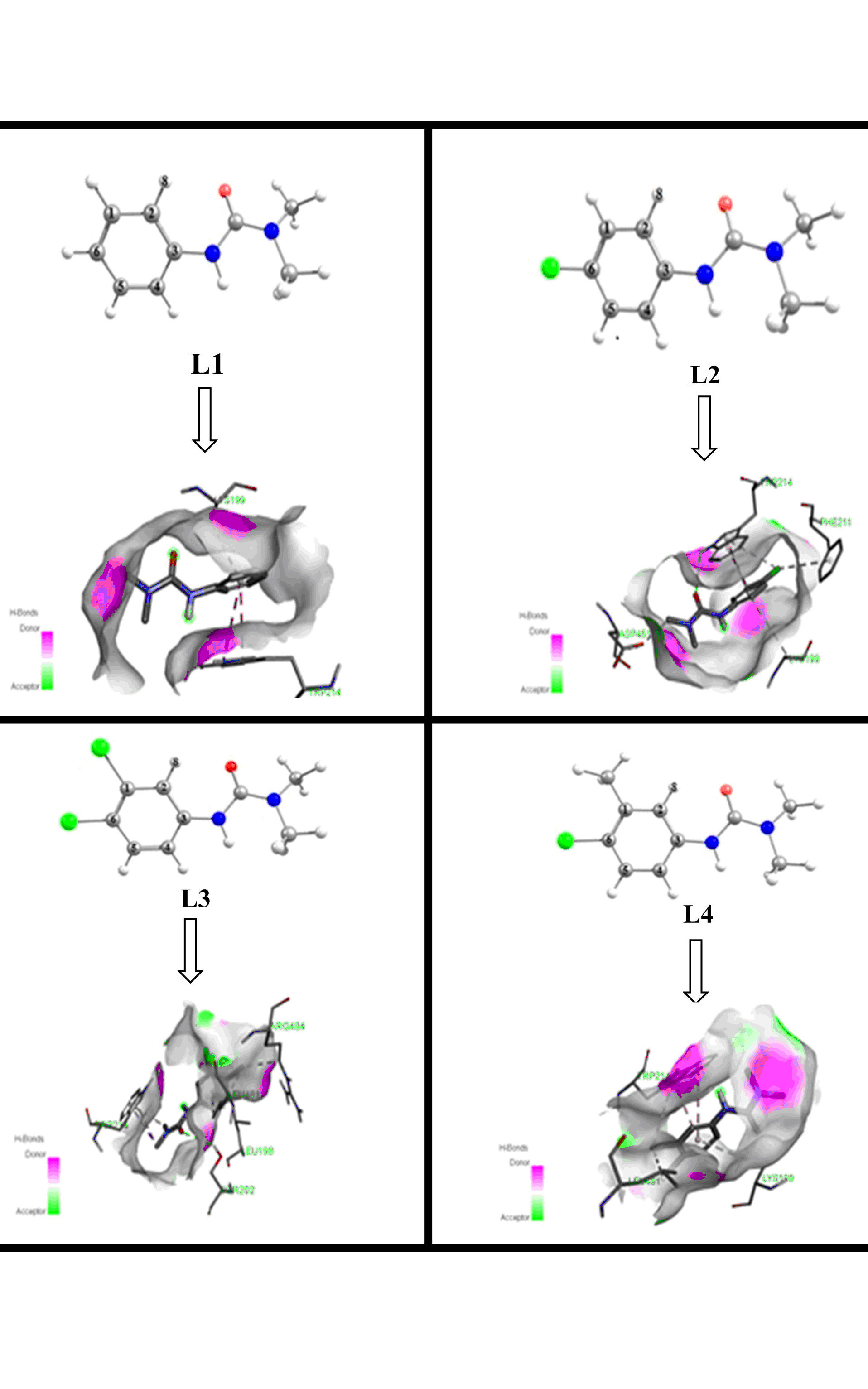
Herbicides have imposed disastrous consequences towards the environment and human health. This practice urges scientists to investigate the physical, chemical and biological properties of these substances to avoid the use of the most harmful pesticides. For this purpose, the molecular structure and chemical bonding properties of phenylurea herbicides namely: fenuron (L1), monuron (L2), diuron (L3) and chlorotoluron (L4), were calculated in water, using density functional theory (DFT). The energy decomposition analysis (EDA) and the extended transition state natural orbitals for chemical valence (ETS-NOCV) reveal the dominant ionic character in carbon–nitrogen bond between dimethylurea fragment and benzene ring. Besides, the interaction of these herbicides with the human serum albumin (HSA) was undertaken by molecular modeling. The calculation of HOMA and FLU indexes indicate that the electronic delocalization is stronger in diuron than the other compounds, mainly caused by the two chloro substituents effects on benzene. Good correlations are found between the calculated parameters such as structural parameters, Mulliken atomic charge, topological and bonding properties and aromaticity indexes. The Vinardo molecular docking results suggest that the binding energies of the complexes formed between HSA target and investigated compounds have the following order: L3 (–27.57 kJ/mol) < L2 (–25.56 kJ/mol) < L4 (–24.94 kJ/mol) < L1 (–24.10 kJ/mol), which confirmed that the Fenuron is the less harmful option between the studied herbicides especially against HSA.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministère de l'Enseignement Supérieur et de la Recherche Scientifique
Grant numbers D01N01UN050220220003
References
P. N. Stamati, S. Maipas, C. Kotampasi, P. Stamatis, L. Hens, Front. Public Health 4 (2016) 1 (https://doi.org/10.3389/fpubh.2016.00148)
H. Mountacer, L. Tajeddine, M. Sarakha, Herbicides and Environment, Intech, Rijeka, 2011 (ISBN 978-953-307-476-4)
R. Kebeish, E. Azab, C. Peterhaensel, R. El-Basheer, Environ. Sci. Pollut. Res. Int. 21 (2014) 8224 (https://doi.org/10.1007/s11356-014-2710-5)
J. Jinhoon, K. Sanjida, M. Youngkook, S. Sooim, O.R. Lee, Appl. Biol. Chem. 63 (2020) 1 (https://doi.org/10.1186/s13765-020-00498-x)
T. Vrabelj, M. Finšgar, Biosensors 12 (2022) 263 (https://doi.org/10.3390/bios12050263)
F. J. Benitez, C. Garcia, J. L. Acero, F. J. Real, World Acad. Sci. Eng. Technol. 34 (2009) 673 (https://doi.org/10.5281/zenodo.1078350)
A. Bautista, J. J. Aaron, M. C. Mahedero and A. Muñoz de la Peña, Analysis 27 (1999) 857 (https://doi.org/10.1051/analusis:1999154)
V. Mile, I. Harsányi, K. Kovács, T. Földes, E. Takács, L. Wojnárovits, Radiat. Phys. Chem. 132 (2017) 16 (https://doi.org/10.1016/j.radphyschem.2016.11.003)
H. Chen, H. Rao, J. Yang, Y. Qiao, J. Yao, J. Environ. Sci. Health., B 51 (2016) 154 (https://doi.org/10.1080/03601234.2015.1108800)
K. Haruna, S. Veena, Y. Kumar, S. A. Sheena Mary, P. R. Thomas, M. S. Roxy, A. A. Al-Saadi, Heliyon 5 (2019) E01987 (https://doi.org/10.1016/j.heliyon.2019.e01987)
L. Humberto, M. Huizar, J. Chem. (2015) 751527 (https://doi.org/10.1155/2015/751527)
F. Zhang, B. Liu, G. Liu, Y. Zhang, J. Wang, S. Wang, Sci. Rep. 8 (2018) 3131 (https://doi.org/10.1038/s41598-018-21394-x)
A. A. Buglak, A. V. Zherdev, H.-T. Lei, B. B. Dzantiev, Plos One 14 (2019) e0214879 (https://doi.org/10.1371/journal.pone.0214879)
Gaussian 09, Gaussian Inc, Wallingford, CT, 2009
A. D. Becke, J. Chem. Phys. 98 (1933) 5648 (https://doi.org/10.1063/1.464913)
A. D. Becke, Phys. Rev., A 38 (1988) 3098 (https://doi.org/10.1103/PhysRevA.38.3098)
C. Lee, W. Yang, and R. G. Parr, Physical Review. B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
F. M. Bickelhaupt, E. J. Baerends, Reviews in computational chemistry, K. B. Lipkowitz, D. B. Boyd, Eds., Wiley-VCH, New York, 2000, pp. 1–86 (https://doi.org/10.1002/9780470125922.ch1)
T. Ziegler, A. Rauk, Inorg. Chem. 18 (1979) 1558 (https://doi.org/10.1021/ic50196a034)
K. B. Wiberg, Tetrahedron 24 (1968) 1083 (https://doi.org/10.1016/0040-4020(68)88057-3)
M. Kohout, Program DGrid, version 4.3, 2008
Chemcraft, Release 1.4 (http://www.chemcraftprog.com)
J. Kruszewski, T. M. Krygowski, Tetrahedron Lett. 13 (1972) 3839 (https://doi.org/10.1016/S0040-4039(01)94175-9)
T. M. Krygowski, J. Chem. Inf. Comp. Sci. 33 (1993) 70 (https://doi.org/10.1021/ci00011a011)
E. Matito, M. Duran, M. Sola, J. Chem. Phys. 122 (2005) (https://doi.org/10.1063/1.1824895)
I. Petitpas, A. A. Bhattacharya, S. Twine, M. East, S. Curry, J. Biol. Chem. 276 (2001) 22804 (https://doi.org/10.1074/jbc.M100575200)
G. Sudlow, D. J. Birkett, Mol. Pharmacol. 12 (1976) 1052
Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, release 2017, Dassault Systèmes, San Diego, CA, 2017
G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell, A. J. Olson, J. Comput. Chem. 30 (2009) 2785 (https://doi.org/10.1002/jcc.21256)
R. Quiroga, M. A. Villarreal, Plos One 11(2016) e0155183 (https://doi.org/10.1371/journal.pone.0155183)
T. Andrew, Mc. Nutt, P. Francoeur, R. Aggarwal, T. Masuda, R. Meli, M. Ragoza, J. Sunseri, D. R. Koes, J. Cheminform. 13 (2021) (https://doi.org/10.1186/s13321-021-00522-2)
T. Gaillard, J. Chem. Inf. Model. (2018) (https://doi.org/10.1021/acs.jcim.8b00312)
D. R. Koes, M. P. Baumgartner, C. J. Camacho, J. Chem. Inf. Model. 53 (2013) 1893 (https://doi.org/10.1021/ci300604z)
O. Trott, A. J. Olson, J. Comput. Chem. 31 (2010) 455 (https://doi.org/10.1002/jcc.21334)
N. Kerru, L. Gummidi, S. V. H. S. Bhaskaruni, S. N. Maddila, P. Singh, B. S. Jonnalagadda, Sci. Rep. 9 (2019) 1 (https://doi.org/10.1038/s41598-019-55793-5)
P. Su, Z. Chen, W. Wu, Chem. Phys. Lett. 635 (2015) 250 (https://doi.org/10.1016/j.cplett.2015.06.078)
K. Shyan, A. Nowroozi, Struct. Chem. 27 (2016) 1769 (https://doi.org/10.1007/s11224-016-0796-8)
S. J. Grabowski, Mol. Struct. 553 (2000) 151 (https://doi.org/10.1016/S0022-2860(00)00576-7)
E. D. Glendening, A. E. Reed, J. E. Carpenter, F. Weinhold, NBO, version 3.1
S. J. Grabowski, Phys. Chem. 102 (2006) 131 (https://doi.org/10.1039/B417200K)
S. Emamian, S. F. Tayyari, J. Chem. Sci. 125 (2013) 939 (https://doi.org/10.1007/s12039-013-0466-y)
G. Mahmoudzadeh, Int. J. New. Chem. 8 (2021) 277 (https://doi.org/10.22034/ijnc.2020.122797.1101)
J. D. Pedelacq, S. Cabantous, T. Tran, T. C. Terwilliger, G. S. Waldo, Nat. Biotechnol. 24 (2006) 79 (https://doi.org/10.1038/nbt1172)
P. V. R. Schleyer, Chem. Rev. 101 (2001) 1115 (https://doi.org/10.1021/cr0103221)
M. K. Cyrañski, Z. Czarnocki, G. Häfelinger, A. R. Katritzky, Tetrahedron 56 (2000) 1783 (https://doi.org/10.1016/S0040-4020(99)00979-5)
J. Poater, M. Duran, M. Solà, B. Silvi, Chem. Rev. 105 (2005) 3911 (https://doi.org/10.1021/cr030085x)
Y. Chen, Y. Zhou, Mo. Chen, B. Xie, J. Yang, J. Chen, Z. Sun, Food Chem. 258 (2018) 393 (https://doi.org/10.1016/j.foodchem.2018.02.105)
H. Xia, Q. Sun, N. Gan, P. Ai, H. Li, Y. Li, RSC Adv. 13 (2023) 8281 (https://doi.org/10.1039/d2ra07377c)
H. Zhang, R. Cai, C. Chen, L. Gao, P. Ding, L. Dai, B. Chi, Int. J. Mol. Sci. 24 (2023) 13281 (https://doi.org/10.3390/ijms241713281)
A. Mahboob, Molecules 28 (2023) 5942 (https://doi.org/10.3390/molecules28165942).