The ethanolic extract of Eryngium billardierei F. Delaroche restrains protein glycation in human serum albumin: An in vitro study Scientific paper
Main Article Content
Abstract
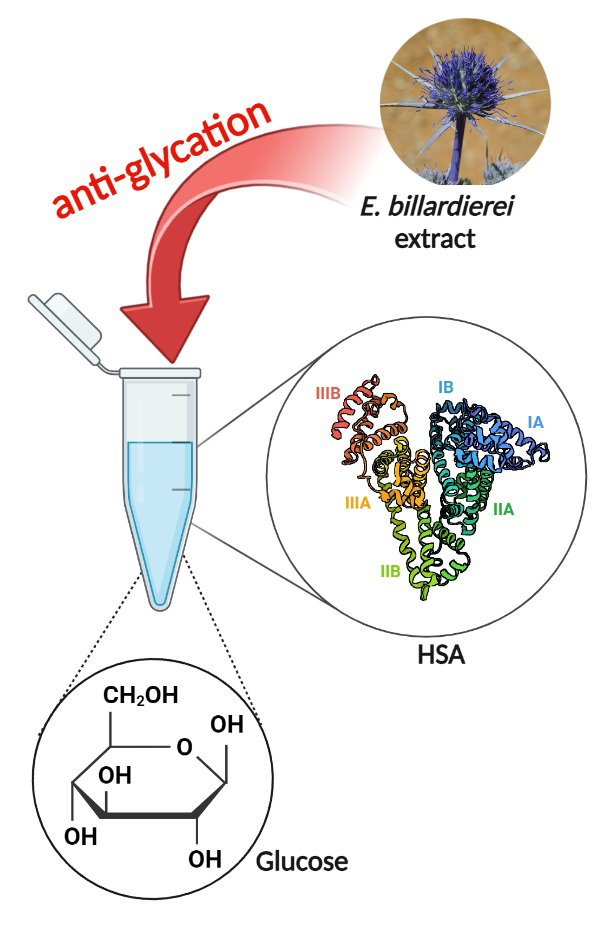
Protein glycation is directly associated with many pathological conditions. This study investigated the potential of Eryngium billardierei extract to inhibit the glycation process in human serum albumin (HSA). After preparation of the ethanolic extract of E. billardierei, the structural changes of glycated HSA in the absence and presence of different concentrations of E. billardierei extract were investigated using circular dichroism (CD), fluorescence spectroscopy and UV–Vis spectroscopy. The results confirmed that E. billardierei extract could reduce the formation of advanced glycation end products (AGEs) and Amadori products under in vitro glycation conditions and also improve HSA helical structure. In addition, a reduction in the HSA-cross amyloid formation was seen in the thioflavin T assay. The phytochemical analysis disclosed that E. billardieri extract is high in flavonoid and phenolic compounds. Accordingly, it could be concluded that the phenolics in E. billardieri extract could prevent glucose-induced HSA glycation. This study provides the rationale that E. billardieri extract could be implicated in controlling diabetes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
G. Rabbani, S. N. Ahn, Int. J. Biol. Macromol. 123 (2019) 979 (https://doi.org/10.1016/j.ijbiomac.2018.11.053)
A. Szkudlarek, A. Sułkowska, M. Maciążek-Jurczyk, M. Chudzik, J. Równicka-Zubik, Spectrochim. Acta, A 152 (2016) 645 (https://doi.org/10.1016/j.saa.2015.01.120)
W. Ge, J. Jie, J. Yao, W. Li, Y. Cheng, W. Lu, Mol. Med. Rep. 25 (2022) 1 (https://doi.org/10.3892/mmr.2022.12656)
E. Sharifi-Zahabi, F. H. Sharafabad, H. Abdollahzad, M. Malekahmadi, N. B. Rad, Adv. Nutr. 12 (2021) 2157 (https://doi.org/10.1093/advances/nmab072)
F. Ahmed, Q. Husain, Biochimie 162 (2019) 66 (https://doi.org/10.1016/j.biochi.2019.04.004)
A. Shamsi, A. Ahmed, M. S. Khan, F. M. Husain, B. Bano, Int. J. Biol. Macromol. 161 (2020) 187 (https://doi.org/10.1016/j.ijbiomac.2020.06.048)
A. Hekmat, S. Hatamie, A. A. Saboury, Inorg. Nano-Met. (2022) 1 (https://doi.org/10.1080/24701556.2022.2034859)
A. Hekmat, R. Bromand Gohar, K. Larijani, J. Med. Herb. 10 (2019) 37 (https://jhd.shahrekord.iau.ir/article_673016.html)
N. Turković, N. Anđelković, D. Obradović, Z. Vujić, B. Ivković, J. Serb. Chem. Soc. 88 (2023) 1 (https://doi.org/10.2298/JSC221212033T)
M. Bohlooli, M. Ghaffari-Moghaddam, M. Khajeh, G. Shahraki-Fallah, B. Haghighi-
-Kekhaiye, N. Sheibani, J. Photochem. Photobiol., B 163 (2016) 345 (https://doi.org/10.1016/j.jphotobiol.2016.09.004)
N. Tran, B. Pham, L. Le, Biology 9 (2020) 252 (https://doi.org/10.3390/biology9090252)
S. Sarmah, A. S. Roy, Int. J. Biol. Macromol. 195 (2022) 565 (https://doi.org/10.1016/j.ijbiomac.2021.12.041)
N. Roshanravan, P. Asgharian, H. Dariushnejad, N. M. Alamdari, B. Mansoori, A. Moh-ammadi, S. Alipour, M. Barati, A. Ghavami, V. Ghorbanzadeh, Adv. Pharm. Bull. 8 (2018) 667 (https://doi.org/10.15171/apb.2018.075)
M. S. Daneshzadeh, H. Abbaspour, L. Amjad, A. M. Nafchi, J. Food Meas. Charact. 14 (2020) 708 (https://doi.org/10.1007/s11694-019-00317-y)
F. Kheirollahzadeh, E. Eftekhari, M. Ghollasi, P. Behzadi, Mol. Biol. Rep. 49 (2022) 3401 (https://doi.org/10.1007/s11033-022-07171-0)
S. Khani, M. Abdollahi, Z. Asadi, M. Nazeri, M. A. Nasiri, H. Yusefi, A. Moghadam, H. Heidari, Res. Pharm. Sci. 16 (2021) 193 (https://doi.org/10.4103/1735-5362.310526)
M. Afshari, A. R. Malayeri, M. Mohammadshahi, J. Contemp. Med. Sci. 5 (2019) 77 (https://doi.org/10.22317/jcms.v5i2.568)
M. K. Siddiqi, P. Alam, S. K. Chaturvedi, S. Nusrat, M. R. Ajmal, A. S. Abdelhameed, R. H. Khan, Int. J. Biol. Macromol. 105 (2017) 292 (https://doi.org/10.1016/j.ijbiomac.2017.07.036)
K. Abdullah, A. Arefeen, A. Shamsi, F. A. Alhumaydhi, I. Naseem, ACS Omega 6 (2021) 12605 (https://doi.org/10.1021/acsomega.1c00631)
F. A. Qais, M. M. Alam, I. Naseem, I. Ahmad, RSC Adv. 6 (2016) 65322 (https://doi.org/10.1039/C6RA12321J)
N. Gligorijević, V. Šukalović, S. Minić, G. Miljuš, O. Nedić, A. Penezić, J. Serb. Chem. Soc. 86 (2021) 795 (https://doi.org/10.2298/JSC210420041G)
H. Schägger, G. Von Jagow, Anal. Biochem. 166 (1987) 368 (https://doi.org/10.1016/0003-2697(87)90587-2)
P. Balyan, M. S. Ola, A. S. Alhomida, A. Ali, Medicina 58 (2022) 1816 (https://doi.org/10.3390/medicina58121816)
M. Jarzębski, P. Siejak, W. Smułek, F. Fathordoobady, Y. Guo, J. Pawlicz, T. Trzeciak, P. Ł. Kowalczewski, D. D. Kitts, A. Singh, Molecules 25 (2020) 2696 (https://doi.org/10.3390/molecules25112696)
R. J. Robbins, J. Agric. Food Chem. 51 (2003) 2866 (https://doi.org/10.1021/jf026182t)
M. Hadidi, A. Garvín, R. Ibarz, A. Ibarz, LWT 154 (2022) 112809 (https://doi.org/10.1016/j.lwt.2021.112809)
N. Na, D.-Q. Zhao, H. Li, N. Jiang, J.-Y. Wen, H.-Y. Liu, Molecules 21 (2015) 54 (https://doi.org/10.3390/molecules21010054)
Y. Wang, X. Wang, J. Wang, Y. Zhao, W. He, Z. Guo, Inorg. Chem. 50 (2011) 12661 (https://doi.org/10.1021/ic201712e)
S. Ahmad, U. Shahab, M. H. Baig, M. S. Khan, M. S. Khan, A. Srivastava, M. Saeed, Moinuddin, PLOS One 8 (2013) e72128 (https://doi.org/10.1371/journal.pone.0072128)
L. Li, Q. Song, X. Zhang, Y. Yan, X. Wang, Molecules 27 (2022) 8793 (https://doi.org/10.3390/molecules27248793)
A. R. Abubakar, M. Haque, J. Pharm. Bioallied Sci. 12 (2020) 1 (https://doi.org/10.4103%2Fjpbs.JPBS_175_19)
L. Gebremeskel, K. Beshir Tuem, T. Teklu, Diabetes, Diabetes Metab. Syndr. Obes. (2020) 1481 (https://doi.org/10.2147/DMSO.S246996)
M. A. Gad-Elkareem, E. H. Abdelgadir, O. M. Badawy, A. Kadri, PeerJ 7 (2019) e6441 (https://peerj.com/articles/6441)
N. Amani, M. Reza Saberi, J. Khan Chamani, Protein Pept. Lett. 18 (2011) 935 (https://doi.org/10.2174/092986611796011473)
A. Ahmed, A. Shamsi, M. S. Khan, F. M. Husain, B. Bano, Int. J. Biol. Macromol. 113 (2018) 269 (https://doi.org/10.1016/j.ijbiomac.2018.02.137)
N. He, R. Wang, Y. He, X. Dang, Sci. China Chem. 55 (2012) 1788 (https://doi.org/10.1007/s11426-012-4604-z)
S. S. Rohiwal, Z. Ellederova, A. P. Tiwari, M. Alqarni, S. T. Elazab, G. E.-S. Batiha, S. H. Pawar, N. D. Thorat, RSC Adv. 11 (2021) 4308 (https://doi.org/10.1039/D0RA09301G)
I. Sirangelo, C. Iannuzzi, Int. J. Mol. Sci. 22 (2021) 6609 (https://doi.org/10.3390/ijms22126609)
K. Abdullah, F. A. Qais, I. Ahmad, H. Hasan, I. Naseem, Int. J. Biol. Macromol. 120 (2018) 1734 (https://doi.org/10.1016/j.ijbiomac.2018.09.176)
M. A. Lambrecht, I. Rombouts, J. A. Delcour, Food Hydrocoll. 57 (2016) 122 (https://doi.org/10.1016/j.foodhyd.2016.01.018)
M. A. B. Siddique, P. Maresca, G. Pataro, G. Ferrari, Food Res. Int. 99 (2017) 419 (https://doi.org/10.1016/j.foodres.2017.06.003)
Y. Feng, X. Ma, B. Kong, Q. Chen, Q. Liu, Food Hydrocoll. 111 (2021) 106379 (https://doi.org/10.1016/j.foodhyd.2020.106379)
P. G. Dorsey, P. Greenspan, J. Med. Food 17 (2014) 447 (https://doi.org/10.1089/jmf.2013.0075).