Geochemistry of neutral mine drainage at sulfide deposits ‒ Example of the „Grot“ Pb–Zn mine, south–eastern Serbia Scientific paper
Main Article Content
Abstract
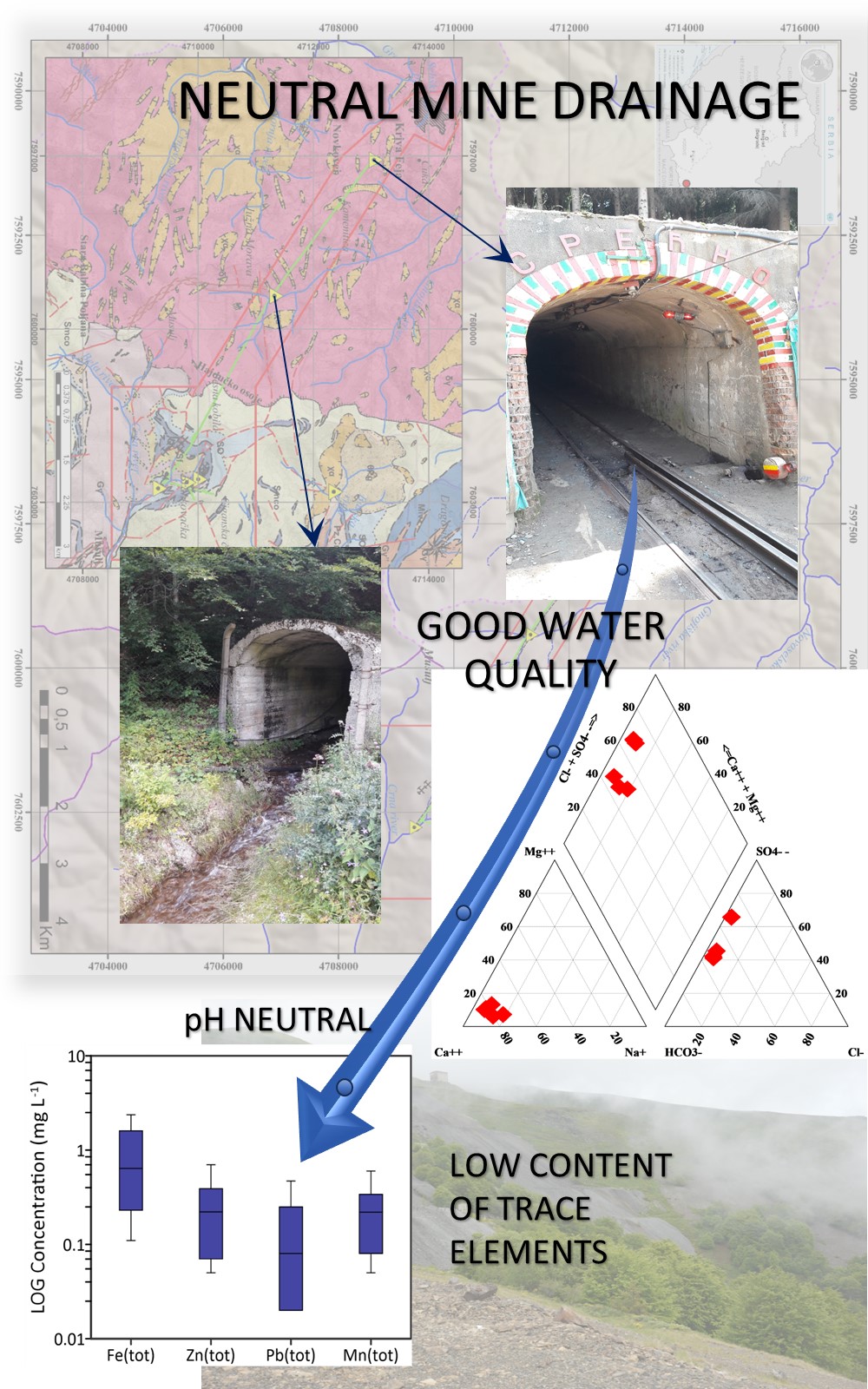
This study examines the chemistry of mine waters of the “Grot” Pb–Zn mine and identifies the hydrogeochemical factors that influence the formation of mine waters chemical composition. Eleven mine water samples were collected at six locations across the area of Kriva Feja in order to determine their chemical composition. Data analysis revealed that the waters belong to the HCO3-‒SO42-‒Ca2+ and SO42-‒Ca2+ water types, with neutral pH values. The concentrations of metals in these waters (zinc, lead, barium, copper, chromium) are generally low, and most of the samples meet drinking water quality criteria (USEPA standards). Modelling using the PHREEQC software indicates that the dominant processes in the formation of the chemical composition of these waters are the dissolution of carbonate minerals and the oxidation of sulphide minerals. Carbonate minerals have a scarcer occurrence compared to sulphide minerals, such as galena, sphalerite and pyrite, which are dominantly distributed. The low intensity of sulphide mineral oxidation is interpreted to result from a rapid water exchange and reduced contact time between the water and the rock. The occurrence of this process is localized only in the ore body zone. This study highlights the importance of kinetics (in terms of the chemical reaction rate) as the main factor influencing the oxidation of sulphide minerals and, subsequently the quality of mine waters.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
C. Wolkersdorfer, D.K. Nordstrom, R. Beckie, D.S. Cicerone, T. Elliot, M. Edraki, T. M. Valente, S. C. A. França, P. Kumar, R.A. Oyarzún Lucero, A. I. G. Soler, Mine Water Environ. 39 (2020) 204 (https://doi.org/10.1007/s10230-020-00666-x)
D. Banks, P. L. Younger, R. T. Arnesen, E. R. Iversen, S.B. Banks, Environ Geol. 32 (1997) 157 (https://doi.org/10.1007/s002540050204)
B.G. Lottermoser, Mine Wastes: Characterization, Treatment and Environmental Impacts Springer, Berlin, 2010, pp. 43–117 (https://doi.org/10.1007/978-3-642-12419-8)
D.K. Nordstrom, Elements 7 (2011) 393 (https://doi.org/10.2113/gselements.7.6.393)
D.K. Nordstrom, C.N. Alpers, In: The environmental geochemistry of mineral deposits, part a: processes, methods, and health issues, G.S. Plumlee, M.J. Logsdon, Eds., Rev Econ Geol, Littleton, 1999, pp. 133–160
A. Parbhakar-Fox, B.G. Lottermoser, Minerals Eng. 82 (2015) 107 (https://doi.org/10.1016/j.mineng.2015.03.015)
O. Guseva, A. K. B. Opitz, J. L. Broadhurst, S. T. L. Harrison, M. Becker, Minerals Eng. 163 (2021) 106750 (https://doi.org/10.1016/j.mineng.2020.106750)
R. Fan, G. Qian, Y. Li, M. D. Short, R. C. Schumann, M. Chen, R. C. Smart, A. R. Gerson, Chem. Geol. 588 (2022) 120653 (https://doi.org/10.1016/j.chemgeo.2021.120653)
N. Atanacković, Risk assessment of water pollution caused by abandoned mining operations in Serbia, Faculty of Mining and Geology, Belgrade, 2018, pp. 23–26 (https://nardus.mpn.gov.rs/handle/123456789/10186)
S. R. Jennings, D. J. Dollhopf, W. P. Inskeep, Appl. Geochem. 15 (2000) 235 (https://doi.org/10.1016/S0883-2927(99)00041-4)
F. P. Walker, M. E. Schreibe, J. D. Rimstidt, Geochim. Cosmochim. Acta 70 (2006) 1668 (https://doi.org/10.1016/j.gca.2005.12.010)
Y. Yunmei, Z. Yongxuan, G. Zheinmin, H. G, Christopher, L. Denxian, Environ. Sci. Tech. 41 (2007) 6460 (https://doi.org/10.1021/es070788m)
G. S. Plumlee, In: The environmental geochemistry of mineral deposits. Part A: Processes, techniques and health issues, G. S. Plumlee, M. S. Logsdon, Eds., Society of Economic Geologists, Littleton, 1999, pp. 77-116
A. P. Jarvis, C. J. Gandy, J. A. Webb, Minerals 13 (2023) 592 (https://doi.org/10.3390/min13050592)
U. O. Chukwura, A. S. Hursthouse, Environ. Earth Sci. 79 (2020) 363 (https://doi.org/10.1007/s12665-020-09108-x)
R. Warrender, N. J. G. Pearce, W. T. Perkins, K. M. Florence, A. R. Brown, D. J. Sapsford, R. J. Bowell, M. Dey, Mine Water Environ. 30 (2011) 82 (https://doi.org/10.1007/s10230-011-0150-8)
N. Barago, E. Pavoni, F. Floreani, M. Crosera, G. Adami, D. Lenaz, S. Covelli, J. Geochem. Explor. 245 (2023) 107129 (https://doi.org/10.1016/j.gexplo.2022.107129)
ISO 5667-1: Water quality - Sampling - Part 1: Guidance on the design of sampling programmes and sampling techniques (2006)
ISO 5667-3: Water quality - Sampling - Part 3: Preservation and handling of water samples (2012)
ISO 5667-10: Water quality — Sampling — Part 10: Guidance on sampling of waste waters (1992)
ISO 10523: Water quality - Determination of pH (2008)
US EPA 160.3: Total Residue by Drying Oven (1983)
ISO 11885: Water quality - Determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES) (2007)
ISO 11969: Water quality - Determination of arsenic - Atomic absorption spectrometric method (hydride technique) (1996)
ISO 7150-1: Water quality - Determination of ammonium - Part 1: Manual spectrometric method (1984)
ISO 7890-3: Water quality - Determination of nitrate - Part 3: Spectrometric method using sulfosalicylic acid (1988)
ISO 6777: Water quality - Determination of nitrite - Molecular absorption spectrometric method (1984)
SRPS H.G8.115: Reagents - Citric acid monohydrate - Determination of sulphate content - Turbidimetric method (1984)
ISO 9297: Water quality - Determination of chloride - Silver nitrate titration with chromate indicator (Mohr’s method) (1989)
ISO 9963-1: Water quality - Determination of alkalinity - Part 1: Determination of total and composite alkalinity (1994)
ISO 5813: Water quality - Determination of dissolved oxygen - Iodometric method (1983)
Drinking Water - Standard Methods for Testing Hygienic Suitability, Federal Institute for Health Protection, Belgrade, 1990
B. J. Merkel, B. Planer-Friedrich, Groundwater Geochemistry. Springer, Berlin, 2005, p. 20
D. L. Parkhurst, C. A. J. Appelo, Description of input and examples for PHREEQC version 3–A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations, Chapter 43 of Section A, Groundwater Book 6, 2013, Modeling Techniques: Techniques and Methods 6–A43
N. Durães, I. Bobos, E. Ferreira de Silva, Environ. Sci. Pollut. Res. 24 (2017) 4562 (https://doi.org/10.1007/s11356-016-8161-4)
J. S. Lee, H. T. Chon, J. Geochem. Expl. 88 (2006) 37 (https://doi.org/10.1016/j.gexplo.2005.08.012)
G. Madzivire, W. M. Gitari, V. R. Kumar Vadapalli, T. V. Ojumu, L. F. Petrik, Min. Eng. 24 (2011) 1467 (https://doi.org/10.1016/j.mineng.2011.07.009)
J. D. Allison, D. S. Brown, K. J. Novo-Gradac, MINTEQA2/PRODEFA2--A geochemical assessment model for environmental systems--version 3.0 user's manual: Environmental Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Athens, GA, 1990, p. 106
USEPA, Drinking water standards and health advisories tables, Office of Water U.S. Environmental Protection Agency Washington, DC, 2018
N. Lilić, D. Knežević, A. Cvjetić, D. Nišić, U. Pantelić, P. Lilić, Environmental impact assessment study for the lead and zinc ore mining project “Vučkovo deposit” and “Kula deposit” within the “Grot” mining company A.D. - Kriva Feja, Belgrade, Faculty of Mining and Geology, Belgrade, 2019, pp. 1–158
N. Atanacković, V. Dragišić, V. Živanović, I. Cvejić, S. Stojadinović, I. Jocić, In: Proceedings of the 16th Serbian Symposium on Hydrogeology with International Participation, Zlatibor, Serbia, 2022, Faculty of Mining and Geology, Belgrade, 2022
ID. Langmuir, Aqueous environmental geochemistry, Prentice Hall, Upper Saddle River, NJ, 1997, pp. 88–90
M. Babović, D. Cvetković, Č. Roglić, V. Avramović, S. Marić, Explanatory book for the basic geologic map, scale 1:100 000, sheet "Trgovište sa Radomir" K 34-57, Institute for Geological and Geophysical Research, Belgrade, 1977, pp. 1–59 (in Serbian).