Synthesis and biological evaluation of some new heterocyclic derivatives from substituted thiopyrimidine Scientific paper
Main Article Content
Abstract
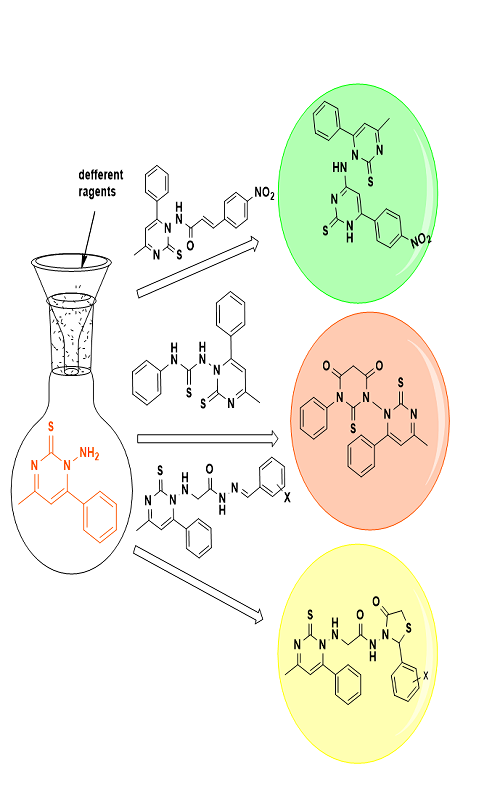
This study aimed at creation of a few new heterocyclic compounds that include sulfur and nitrogen atoms. Also, some chalcones, thiazolidine and Schiff base derivatives have been prepared. The spectroscopic data (IR, 1H- and 13C-NMR) have verified the structure of the produced molecules. Interesting findings were obtained when several synthetic substances were physiologically tested against a range of pathogenic Gram-positive and Gram-negative bacteria.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
N. N. Makhova, L. I. Belen’kii, G. A. Gazieva, I. L. Dalinger, L. S. Konstantinova, V. V. Kuznetsov, A. N. Kravchenko, M, M. Krayushkin, O. A. Rakitin, A. M. Starosotnikov, L. L. Fershtat, S. A. Shevelev, V. Z. Shirinian, V. N. Yarovenko, Russ. Chem. Rev. 89 (2020) 55 (https://dx.doi.org/10.1070/RCR4914)
A. Z. A. Tlais, G. M. Fiorino, A. Polo, P. Filannino, R. Di Cagno, Molecules 25 (2020) 2987 (https://dx.doi.org/10.3390/molecules25132987)
H. Kumari, H. Kumar, K. Sharma, IARS’ Int. Res. J. 12 (2023) 1839 (https://dx.doi.org/10.51611/iars.irj.v12i02.2022.213)
M. M. Heravi, V. Zadsirjan, RSC Adv. 10 (2020) 44247 (https://dx.doi.org/10.1039/D0RA09198G)
M. N. Patel, P. S. Karia, P. A. Vekariya, A. P. Patidar. Arab. J. Chem. 12 (2019) 2983 (https:/dx./doi.org/10.1016/j.arabjc.2015.06.031)
A. Lattanzi, Chem. Rec. 23 (2023) 1 (https://dx.doi.org/10.1002/tcr.202300066)
R. Arivazhagan, C. Sridevi, A. Prakasam, J. Mol. Struct. 1232 (2021) 129956 (https://dx.doi.org/10.1016/j.molstruc.2021.129956)
E. Zarenezhad, M. Farjam, A. Iraji, J. Mol. Struct. 1230 (2021) 129833 (https://doi.org/10.1016/j.molstruc.2020.129833)
S. Bhilare, H. Shet, Y. S. Sanghvi, A. R. Kapdi, Molecules 25 (2020) 1645 (https://dx.doi.org/10.3390/molecules25071645)
W. K. Olson, S. Li, T. Kaukonen, A. V. Colasanti, Y. Xin, X. J. Lu, Biochemistry 58 (2019) 2474 (https://dx.doi.org/10.1021/acs.biochem.9b00122)
Á. Ramírez-Trinidad, K. Carrillo-Jaimes, J. A. Rivera-Chávez, E. Hernández-Vázquez, Med. Chem. Res. 32 (2023) 144 (https://dx.doi.org/10.1007/s00044-022-02997-6)
S. Singh, S. Ahmad, D. Mehta, S. Alam, Pharm. Sci. Technol. 3 (2019) 40 (https://dx.doi.org/10.11648/j.pst.20190302.12)
P. Srivastava, G. Teli, P. A. Chawla, Lett. Drug Des. Discov. 20 (2023) 894 (https://dx.doi.org/10.2174/1570180819666220523142245)
N. Long, A. Le Gresley, S. P. Wren, ChemMedChem 16 (2021) 1717 (https://dx.doi.org/10.1002/cmdc.202100177)
N. Trotsko, J. Golus, P. Kazimierczak, A. Paneth, A. Przekora, G. Ginalska, M. Wujec, Eur. J. Med. Chem. 189 (2020) 112045 (https://dx.doi.org/10.1016/j.ejmech.2020.112045)
S. R. Atta-Allah, N. S. M- Ismail, I. F. Nassar, Lett. Drug Des. Discov. 18 (2021) 525 (https://dx.doi.org/10.2174/1570180817999201123164201)
M. Rashid, N. Shrivastava, A. Husain, J. Chilean Chem. Soc. 65 (2020) 4817 (http://dx.doi.org/10.4067/S0717-97072020000204817)
A. Amin, T. Qadir, A. Salhotra, P. K. Sharma, I. Jeelani, H. Abe, Curr. Bioact. Compd. 18 (2022) 77 (https://dx.doi.org/ 10.2174/1573407218666220303100501)
S. Rocha, A. Sousa, D. Ribeiro, C. M. Correia, V. L. M. Silva, C. M. M. Santos, A. M. S. Silva, A. N. Araújo, E. Fernandes, M. Freitas, Food Funct. 10 (2019) 5510 (https://dx.doi.org/10.1039/C9FO01298B)
M. C. Egbujor, S. Saha, B. Buttari, E. Profumo, L. Saso. Expert Rev. Clin. Pharmacol. 14 (2021) 465 (https://dx.doi.org/10.1080/17512433.2021.1901578)
M. Rudrapal, J. Khan, A. A. Bin Dukhyil, R. M. I. I. Alarousy, E. I. Attah, T. Sharma, S. J. Khairnar, A. R. Bendale, Molecules 26(2021) 7177 (https://dx.doi.org/10.3390/molecules26237177)
R. de A. M. Neto, C. B. R. Santos, S. V. C. Henriques, L. de O. Machado, J. N. Cruz, C. H. T. de P. da Silva, a Silva, L. B. Federico, E. H. C. de Oliveira, M. P. C. de Souza, P. N. B. da Silva, C- A. Tafte, I, M. Ferreira, M, R. F. Gomes, J. Biomol. Struct. Dyn. 40 (2022) 2204 (https://dx.doi.org/10.1080/07391102.2020.1839562)
M. A. El-Hashash, S. A. Rizk, S. R. Atta-Allah, Molecules 20 (2015) 22069 (https://dx.doi.org/10.3390/molecules201219827)
A. A. Ahmed, I. Q. Mahmood, H. S. Aziz. Int, J. Drug Delivery Technol. 12 (2022) 1087 (https://dx.doi.org/10.25258/ijddt.12.3.27)
A. Mermer, N. Demirbas, H. Uslu, A. Demirbas, S. Ceylan, Y. Sirin, J. Mol. Struct. 1181 (2019) 412 (https://dx.doi.org/10.1016/j.molstruc.2018.12.114)
S. M. Gomha, H. A. Ahmed, M. Shaban, T. Z. Abolibda, M. S. Khushaim, K. A. Alharbi, Materials 14 (2021) 3718 (https://dx.doi.org/10.3390/ma14133718)
A. A. Hamed, I. A. Abdelhamid, G. R. Saad, N. A. Elkady, M. Z. Elsabee, Int. J. Biol. Macromol. 153 (2020) 492 (https://dx.doi.org/10.1016/j.ijbiomac.2020.02.302)
I. Q. M. Alaraj, R. A. Saeed, L. Reyadh, A. A. Ahmed, J. Turkish Chem. Soc. Sect. Chem. 11 (2024) 425 (https://dx.doi.org/10.18596/jotcsa.1371936)
A. A. Ahmed, N. G. Ahmed, A. K. Ahmad. Pak. J. Sci. Ind. Res. Ser. A: Phys. Sci. 63 (2020) 1 (https://dx.doi.org/10.52763/PJSIR.PHYS.SCI.63.1.2020.1.11)
S. A. Abdul Husseina, A. A.M. Kubbab, Der. Pharma. Chem. 7 (2015) 250 (https://www.derpharmachemica.com/pharma-chemica/synthesis-characterization-and-antimicrobial-activity-of-new-25disubstituted134thiadiazole-derivatives.pdf)
K. K. Bedia, O. Elçin, U. Seda, K. Fatma, S. Nathaly, R. Sevim, A. Dimoglo, Eur. J. Med. Chem. 41 (2006) 1253 (https://dx.doi.org/10.1016/j.ejmech.2006.06.009)
S. Senthilkumar, J. Seralathan, G. Muthukumaran, J. Mol. Struct. 1226 (2021) 129354 (https://dx.doi.org/10.1016/j.molstruc.2020.129354)
M. A. Gouda, A. A. Abu-Hashem. Arch. Pharm. (Weinheim) 344 (2011) 170 (https://dx.doi.org/10.1002/ardp.201000165)
M. Amir, K. Shikha, Eur. J. Med. Chem. 39 (2004) 535 (https://dx.doi.org/10.1016/j.ejmech.2004.02.008)
A. A. Aly, R. El-Sayed, Chem. Papers 60 (2006) 56 (https://dx.doi.org/10.2478/s11696-006-0010-3)
T. A. Farghaly, G. S. Masaret, Z. A. Muhammad, M. F. Harras, Bioorg. Chem. 98 (2020) 103761 (https://dx.doi.org/10.1016/j.bioorg.2020.103761)
P. Ngamsurach, P. Praipipat, RSC Adv. 12 (2022) 26435 (https://doi.org/10.1039/D2RA04611C)
K. Omar, A. Geronikaki, P. Zoumpoulakis, C. Camoutsis, M. Soković, A. Ćirić, J. Glamočlija, Bioorg. Med. Chem. 18 (2010) 426 (https://dx.doi.org/10.1016/j.bmc.2009.10.041)
T. Chaban, Y. Matiichuk, Z. Chulovska, O. Tymoshuk, I. Chaban, V. Matiychuk, Arch. Pharm. 354 (2021) 2100037 (https://dx.doi.org/10.1002/ardp.202100037)
C. J. Galvin, M. Hobson, J. X. Meng, A. Ierokomos, J. Biol. Chem. 299 (2023) 103003 (https://dx.doi.org/10.1016/j.jbc.2023.103003)
C. Tratrat, A. Petrou, A. Geronikaki, M. Ivanov, M. Kostić, M. Soković, I. S. Vizirianakis, N. F. Theodoroula, M. Haroun, Molecules 27 (2022) 1930 (https://dx.doi.org/10.3390/molecules27061930).