Synthesis of and theoretical research on some azine derivatives and investigation of their antimicrobial activities Scientific paper
Main Article Content
Abstract
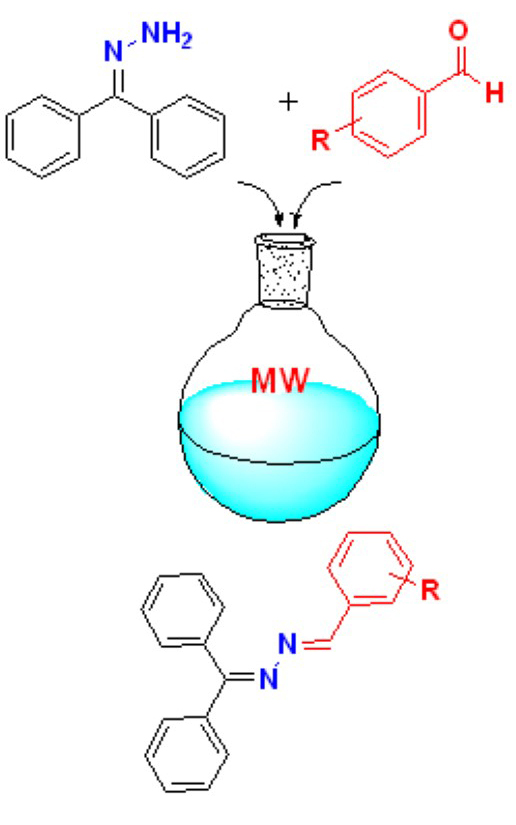
This study includes experimental, theoretical, and antimicrobial investigations on 1-(diphenylmethylene)-2-(4-methoxybenzylidene)hydrazine (5), 1-(3,5-dimethoxybenzylidene)-2-(diphenylmethylene)hydrazine (6) and 1-(diphenylmethylene)-2-(2,3,4-trimethoxybenzylidene)hydrazine (7). The structures of the compounds synthesized by microwave method were determined by spectroscopic methods and elemental analysis. Conformational analysis, ground state structure, fourier transform infrared spectra (FT-IR), and nuclear magnetic resonance (NMR) spectra of the compounds were computed using density functional theory (DFT) calculations in the theoretical research. Based on the B3LYP/6-31G(d,p) level, the conformers from the torsional barrier scanning were optimized. The B3LYP/6-311++G .(d,p) was used to determine the harmonic vibrational frequencies, potential energy distribution (PED), infrared intensities, and NMR chemical shifts of the most stable conformers. The experimental findings were compared with theoretically expected spectral data. The antibacterial activity of the prepared compounds was tested in vitro against nine bacteria and one yeast species. The antimicrobial activity of the compounds was tested by minimum inhibitory concentration (MIC) and agar well diffusion method. Compound 7 showed good activity against the bacteria and yeast, while 5 and 6 showed no antimicrobial activity. Compound 7 showed zone of inhibition values in the range of 10-15 mm against Klebsiella pneumonia, Pseudomonas aeruginosa and Salmonella typhimurium The results indicated that compound 7 was effective against bacteria.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
D. Amariucai-Mantu, V. Mangalagiu, I. Bejan, A. Aricu and II. Mangalagiu, Pharmaceutics. 2 (2022) 2026 (https://doi.org/10.3390/pharmaceutics14102026)
M. F. N. N. Carvalho, Antibiotics. 11 (2022) 337 (https://doi.org/10.3390/antibiotics11030337)
A. S. Salman, N. A. Mahmoud, A. Abdel-Aziem, M. A. Mohamed and D. M. Elsisi, Int J Org Chem. 5 (2015) 81 (https://doi.org/10.4236/ijoc.2015.52010 )
S. Kulaksızoglu, C. Gökçe, R. Gup, Turk. J. Chem. 36 (2002) 717 (https://doi.org/10.3906/kim-1110-3)
S. P. Simeonov, V. B. Kurteva, R. P. Bontchev, Bulg. Chem. Commun. 40 (2008) 409 (http://www.bcc.bas.bg/bcc_volumes/Volume_40_Number_4_2008/Volume_40_Number_4_2008_PDF/BCC_v40_n4.pdf#page=39)
B. Krishnakumar and M. Swaminathan, Catal. Commun. 16 (2011) 50 (https://doi.org/10.1016/j.molcata.2011.08.026)
H. Loghmani-Khouzani, M. M. M. Sadeghi, J. Safari, M. S. Abdorrezaie and M. Jafarpisheh, J. Chem. Research 2 (2001) 80 (https://doi.org/10.3184/030823401103169036)
K. Venkatesan, V. S. V. Satyanarayana, A. Sivakumar, J. Chin. Chem. Soc. 58 (2011) 583 (https://doi.org/10.1002/jccs.201190091)
A. Y. Vibhute, S. S. Mokle, Y. S. Nalwar, Y. B. Vibhute, V. M. Gurav, Bulletin of the Catalysis Society of India 8 (2009) 164 (https://www.researchgate.net/profile/Shyam-Mokle/publication/257656004_An_Efficient_and_Operationally_Simple_Synthesis_of_Some_New_Schiff_Bases_Using_Grinding_Technique/links/00463525af8a3c66e6000000/An-Efficient-and-Operationally-Simple-Synthesis-of-Some-New-Schiff-Bases-Using-Grinding-Technique.pdf)
W. A. A. Arafa, R. M. Shaker, ARKIVOC (iii) (2016)187 (http://dx.doi.org/10.3998/ark.5550190.p009.464)
A. Rammohan, J. S. Reddy, G. Sravya, C. N. Rao, G. V. Zyryanov, Environ. Chem. Lett. 18 (2002) 433 (https://doi.org/10.1007/s10311-019-00959-w)
S. Aytaç, JIST. 11 (2021) 2979-2991 (https://doi.org/10.21597/jist.976184)
Safari, S. Gandomi-Ravandi, Synth. Commun. 41 (2011) 645 (https://doi.org/10.1080/00397911003629523)
J. Jayabharathi, V. Thanikachalam, A. Thangamani, M. Padmavathy, Med Chem Res. 16 (2007) 266 (https://doi.org/10.1007/s00044-007-9029-4)
A. Zieba, Z. P. Czuba and W. Krol, Acta Pol. Pharm. 69 (2012) 1149 (https://www.ptfarm.pl/pub/File/Acta_Poloniae/2012/6/1149.pdf)
H. Çelik, A. Babagil, Int. J. Second. Metab. 6 (2019) 38 (https://doi.org/10.21448/ijsm.479108)
M. Ayaz, O. Gundogdu, S. Aytac, B. Erdem, H. Ciftci, Y. Erdogdu, J. Mol. Struct. 1269 (2022) 133791 (https://doi.org/10.1016/j.molstruc.2022.133791)
K. Ravi, B. Krishnakumar, M. Swaminathan, Int. Sch. Res Notices. (2012) 595868 (https://doi.org/10.5402/2012/595868)
M. J. Frisch et al. Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT (2016)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B37 (1988) 785 (https://doi.org/10.1103/physrevb.37.785)
A. D. Becke, Phys. Rev. A. 38 (1988) 3098 (https://doi.org/10.1103/PhysRevA.38.3098)
A. D. Becke, J. Chem. Phys. 98 (1993) 5648 (https://doi.org/10.1063/1.464913)
D. Sajan, Y. Erdogdu, T. Kuruvilla, I. H. Joe, J. Mol. Struct. 983 (2010) 12 (https://doi.org/10.1016/j.molstruc.2010.08.003)
Spartan 08, Wavefunction Inc., Irvine, CA 92612, USA (2008) ISBN978-1-890661-38-4
T. A. Halgren, J. Comput. Chem. 17 (1996) 490 (https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P)
Y. Erdogdu, Ö. Dereli, D. Sajan L. Joseph, O. Ünsalan, M. T. Güllüoğlu, Mol. Simul. 38 (2012) 315 (https://doi.org/10.1080/08927022.2011.632416)
S. Saglam, A. Disli, Y. Erdogdu, M. K. Marchewka, N. Kanagathara, B. Bay, Spectrochim. Acta A Mol. Biomol. Spectrosc. 135 (2015)1011 (https://doi.org/10.1016/j.saa.2014.07.071)
A. Borba, M. Albrecht, A. G. Zavaglia, L. Lapinski, M. J. Nowak, M. A. Suhmb, R. Fausto, Phys. Chem. Chem. Phys. 10 (2008) 7010 (https://doi.org/10.1039/B810002K)
A. Atilgan, S. Yurdakul, Y. Erdogdu, M. T. Gulluoglu, J. Mol. Struct. 1161 (2018) 55 (https://doi.org/10.1016/j.molstruc.2018.01.080)
Y. Erdogdu, S. Saglam, M. T. Gulluoglu, Spectrochim. Acta A Mol. Biomol. Spectrosc.146, (2015) 88 (https://dx.doi.org/10.1016/j.saa.2015.03.031)
M. Yilmaz, B. Aydin, O. Dogan, O. Dereli, J. Mol. Struct. 1128 (2017) 345 (https://doi.org/10.1016/j.molstruc.2016.08.067)
E. K. Sarıkaya, S. Bahçeli. D. Varkal, O. Dereli, J. Mol. Struct. 1141 (2017) 44 (https://doi.org/10.1016/j.molstruc.2017.03.088)
Y. Erdogdu, Ş. Yurdakul, S. Badoglu, M.T. Güllüoğlu, J. Mol. Struct. 1184 (2019) 364 (https://doi.org/10.1016/j.molstruc.2019.02.016)
S. Chandra, H. Saleem, Y. Erdogdu, S. Subashchandrabose, A. R. Krishnan, M. T. Güllüoğlu, J. Mol. Struct. 998 (2011) 69 (https://doi.org/10.1016/j.molstruc.2011.05.014)
S. Çelik, M. Alp, S. Yurdakul, Spectrosc. Lett. 53 (2020) 234 (https://doi.org/10.1080/00387010.2020.1734840)
S. Çelik, S. Yurdakul, B. Erdem, Inorg. Chem. Commun. 131 (2021)108760 (https://doi.org/10.1016/j.inoche.2021.108760)
Ö. Dereli, Y. Erdogdu, M. T. Gulluoglu, E. Türkkan, A. Özmen, N. Sundaraganesan, J. Mol. Struct. 1012 (2012) 168 (https://doi.org/10.1016/j.molstruc.2012.01.003)
L. Joseph, D. Sajan, R. Reshmy, B. S. A. Sasi, Y. Erdogdu, K. K. Thomas, Spectrochim. Acta A Mol. Biomol. Spectrosc. 99 (2012) 234 (https://doi.org/10.1016/j.saa.2012.07.084)
N. R. Babu, S. Subashchandrabose, M. S. A. Padusha, H. Saleem, Y. Erdogdu, Spectrochim. Acta A Mol. Biomol. Spectrosc. 120 (2014) 314 (https://doi.org/10.1016/j.saa.2013.09.089)
H. Z. Shams, R. M. Mohareb, M. H. Helal, A. E-S. Mahmoud, Molecules 16 (2011) 6271 (https://doi.org/10.3390/molecules16086271)