The cyanide, cyanate, thiocyanate ambident anions: Structure, topological analysis of electron density and homolytic oxidative coupling regioselectivity Scientific paper
Main Article Content
Abstract
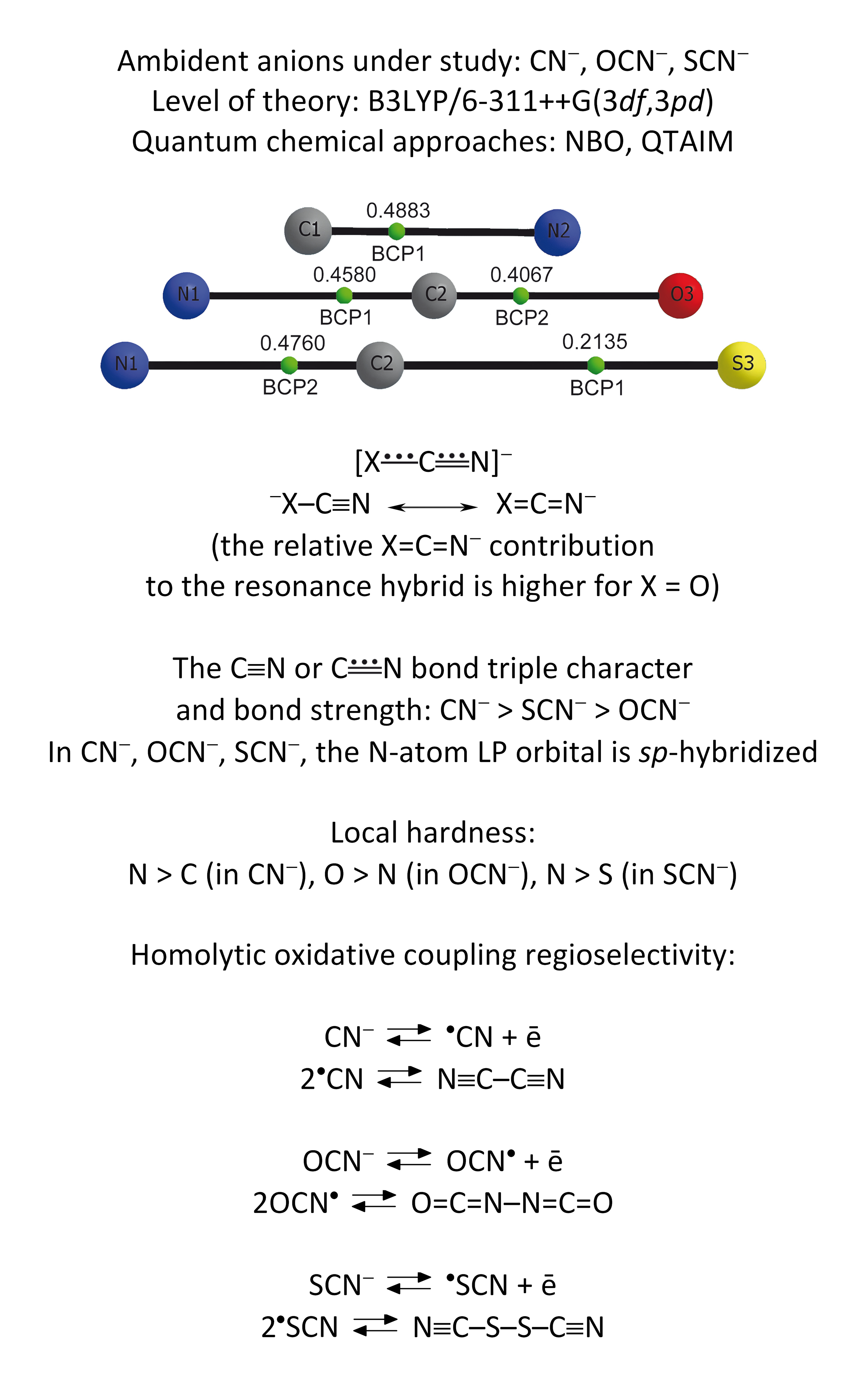
At the B3LYP/6-311++G(3df,3pd) level of theory, the spatial and electronic structure of the cyanide, cyanate and thiocyanate ambident anions has been studied. By means of the natural bond orbital (NBO) analysis and the R. F. W. Bader’s quantum theory “Atoms in Molecules” (QTAIM), the electron density delocalization and topological properties in the above anions have been investigated. The distribution of electron density (NBO, QTAIM) in the XCN- (X = O, S) anions is reflected by the scheme 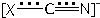 . The relative contribution of the hypothetical structure X=C=N- to the resonance hybrid -X–C≡N ↔X=C=N- is higher in the case of X = O. The degree of the C≡N or
. The relative contribution of the hypothetical structure X=C=N- to the resonance hybrid -X–C≡N ↔X=C=N- is higher in the case of X = O. The degree of the C≡N or  bond triple character and bond strength changes in the following series of anions: CN- > SCN- > OCN-. The occupancy of the lone electron pair (LP) orbital of the nitrogen atom in the above anions is close to 2, and the LP orbital is sp-hybridized. Condensed K. Fukui functions for the electrophilic attack have been evaluated. Local hardness of the donor reaction centres: N > C (CN-), O > N (OCN-), N > S (SCN-). The regioselectivity of the homolytic oxidative coupling reactions of the CN-, OCN-, SCN- anions has been substantiated.
bond triple character and bond strength changes in the following series of anions: CN- > SCN- > OCN-. The occupancy of the lone electron pair (LP) orbital of the nitrogen atom in the above anions is close to 2, and the LP orbital is sp-hybridized. Condensed K. Fukui functions for the electrophilic attack have been evaluated. Local hardness of the donor reaction centres: N > C (CN-), O > N (OCN-), N > S (SCN-). The regioselectivity of the homolytic oxidative coupling reactions of the CN-, OCN-, SCN- anions has been substantiated.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. E. House, Inorganic Chemistry, Academic Press (imprint of Elsevier Inc.), Amsterdam, 2008 (ISBN: 978-0-12-356786-4)
G. L. Miessler, P. J. Fischer, D. A. Tarr, Inorganic Chemistry, Pearson Education, Inc. (Pearson advanced chemistry series), Boston, MA, 2014 (ISBN-10: 0321811054; ISBN-13: 978-0-321-81105-9)
R. K. Sharma, Text Book of Coordination Chemistry, Discovery Publishing House, New Delhi, 2007 (ISBN-10: 81-8356-223-X; ISBN-13: 978-8183562232)
M. H. Najar, A Comprehensive Guide to Coordination Chemistry, Spectra and Magnetism: Frontiers of Inorganic Chemistry, LAP LAMBERT Academic Publishing, Saarbrücken, 2020 (ISBN-10: 6202516321; ISBN-13: 978-6202516327)
B. Weber, Coordination Chemistry: Basics and Current Trends, Springer Spektrum, Berlin, 2023 (https://doi.org/10.1007/978-3-662-66441-4)
Theoretical Organic Chemistry, C. Párkányi, Ed., Elsevier Science, Amstrdam, 2011 (ISBN: 978-0444546227)
P. Vogel., P. N. Houk, Organic Chemistry: Theory, Reactivity and Mechanisms in Modern Synthesis, Wiley-VCH Verlag GmbH, Weinheim, 2019 (ISBN: 978-3-527-81927-0)
R. G. Pearson, Chemical Hardness: Applications from Molecules to Solids, Wiley-VCH Verlag GmbH, Weinheim, 1997 (https://doi.org/10.1002/3527606173)
A. A. Tishkov, H. Mayr, Angew. Chem. Int. Ed. 44 (2005) 142 (https://doi.org/10.1002/anie.200461640)
H. F. Schaller, U. Schmidhammer, E. Riedle, H. Mayr, Chem. – Eur. J. 14 (2008) 3866 (https://doi.org/10.1002/chem.200800314)
R. Loos, Sh. Kobayashi, H. Mayr, J. Amer. Chem. Soc. 125 (2003) 14126 (https://doi.org/10.1021/ja037317u)
A. M. Golub, H. Kőhler, V. V. Skopenko, H. Boland, T. P. Lishko, V. M. Samoilenko, G. V. Tsintsadze, Chemistry of Pseudohalides, A. M. Golub, H. Kőhler, V. V. Skopenko, Eds., Elsevier Science Ltd, Amsterdam, 1986 (ISBN-10: 0444416269; ISBN-13: 9780444416261)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, John Wiley & Sons, Inc., Hoboken, NJ, 2009 (https://doi.org/10.1002/9780470405840)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part B: Application in Coordination, Organometallic, and Bioinorganic Chemistry, John Wiley & Sons, Inc., Hoboken, NJ, 2009 (https://doi.org/10.1002/9780470405888)
A. F. Wells, Structural Inorganic Chemistry, Oxford Univ. Press, Oxford, 2012 (ISBN-10: 0199657637; ISBN-13: 978-0199657636)
G. P. Mikhailov, J. Appl. Spectrosc. 83 (2016) 339 (https://doi.org/10.1007/s10812-016-0305-2)
A. N. Pankratov, O. M. Tsivileva, Current Phys. Chem. 6 (2016) 210 (https://doi.org/10.2174/1877946806666160524154445)
F. M. Fernández, Introduction to Perturbation Theory in Quantum Mechanics, CRC Press, Inc., Boca Raton, FL, 2000 (https://doi.org/10.1201/9781420039641)
R. V. Selezenev, Psevdogalogeny i ikh soedineniya Pseudohalogens and Their Compounds, https://present5.com/psevdogalogeny-i-ix-soedineniya-selezenev-r-v/ (accessed 7th December 2023)
A. N. Pankratov, J. Mol. Struct.: THEOCHEM 315 (1994) 179 (https://doi.org/10.1016/0166-1280(94)03779-K)
A. N. Pankratov, A. N. Stepanov, Croat. Chem. Acta 70 (1997) 585 (ISSN: 0011-1643; e-ISSN: 1334-417X)
A. N. Pankratov, J. Anal. Chem. 77 (2022) 1063 (https://doi.org/10.1134/S106193482209009X)
I. N. Levine, Quantum Chemistry, Pearson, Boston, MA, 2014 (ISBN-10: 0321803450; ISBN-13: 978-0321803450)
W. Kohn, Rev. Mod. Phys. 71 (1999) 1253 (https://doi.org/10.1103/RevModPhys.71.1253)
W. Koch, M. C. Holthausen, Chemist’s Guide to Density Functional Theory, Willey-VCH Verlag GmbH, Toronto, 2001 (https://doi.org/10.1002/3527600043)
S. F. Sousa, P. A. Fernandes, M. J. Ramos, J. Phys. Chem., A 111 (2007) 10439 (https://doi.org/10.1021/jp0734474)
Gaussian 09, Revision E.01, Gaussian, Inc., Wallingford, CT, 2013 https://gaussian.com (accessed 7th December 2023)
A. D. Becke, Phys. Rev. A 38 (1988) 3098 (https://doi.org/10.1103/physreva.38.3098)
A. D. Becke, J. Chem. Phys. 98 (1993) 5648 (https://doi.org/10.1063/1.464913)
Chengteh Lee, Weitao Yang, R. G. Parr, Phys. Rev., B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
H. B. Schlegel, J. J. W. McDouall, in Computational Advances in Organic Chemistry, C. Ögretir, I. G. Csizmadia, Eds., Kluwer Academic, Dordrecht, 1991, pp. 167–185 (https://doi.org/10.1007/978-94-011-3262-6)
R. Krishnan, J. S. Binkley, R. Seeger, J. A. Pople, J. Chem. Phys. 72 (1980) 650 (https://doi.org/10.1063/1.438955)
A. D. McLean, G. S. Chandler, J. Chem. Phys. 72 (1980) 5639 (https://doi.org/10.1063/1.438980)
HyperChem Professional 8.0, Hypercube, Inc., Gainesville, FL, http://hypercubeusa.com/Products/HyperChemProfessional/tabid/360/Default.aspx; http://www.hypercubeusa.com/Products/HyperChemProfessional/tabid/360/Default.aspx (accessed 7th December 2023)
J. J. P. Stewart, J. Comput. Chem. 10 (1989) 209 (https://doi.org/10.1002/jcc.540100208)
J. J. P. Stewart, J. Comput. Chem. 10 (1989) 221 (https://doi.org/10.1002/jcc.540100209)
A. E. Reed, L. A. Curtiss, F. Weinhold, Chem. Rev. 88 (1988) 899 (https://doi.org/10.1021/cr00088a005)
A. V. Nemukhin, F. Weinhold, Ros. Khim. Zh. (Zh. Ros. Khim. Obshchestva im. D. I. Mendeleeva 38 (1994) 5 (ISSN: 0373-0247)
F. Weinhold, C. R. Landis, Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective, Cambridge Univ. Press, Cambridge, 2005 (https://doi.org/10.1017/CBO9780511614569)
I. Mayer, Bond Orders and Energy Components: Extracting Chemical Information from Molecular Wave Functions, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2016 (https://doi.org/10.1201/9781315374895)
NBO Version 3.1, 1995, https://gaussian.com (accessed 7th December 2023)
R. F. W. Bader, in Chemical Applications of Topology and Graph Theory: A Collection of Papers from a Symposium Held at the Univ. of Georgia. Athens, Georgia, USA, 18-22 April, 1983, R. B. King, Ed., Elsevier Science Ltd, Amsterdam, 1983, pp. 40–56 (ISBN-10: 0444422447; ISBN-13: 978-0444422446)
D. Cremer, E. Kraka, Croat. Chem. Acta 37 (1984) 1259 (ISSN: 0011-1643; e-ISSN: 1334-417X)
R. F. W. Bader, Acc. Chem. Res. 18 (1985) 9 (https://doi.org/10.1021/ar00109a003)
R. F. W. Bader, Pure Appl. Chem. 60 (1988) 145 (https://doi.org/10.1351/pac198860020145)
R.F.W. Bader, Chem. Rev. 91 (1991) 893 (https://doi.org/10.1021/cr00005a013)
R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Oxford Univ. Press, Clarendon Press, New York, 1994 (ISBN-10: 0198551681; ISBN-13: 978-0198551683)
R. F. W. Bader, P. L. A. Popelier, T. A. Keith, Angew. Chem. 106 (1994) 647 (https://doi.org/10.1002/ange.19941060605)
P. L. A. Popelier, Atoms in Molecules: An Introduction, Prentice Hall, London, 2000 (ISBN-10: 0582367980; ISBN-13: 978-0582367982)
P. L. A. Popelier, F. M. Aicken, S. E. O’Brien, in Chemical Modelling: Applications and Theory. Vol. 1, Ch. A. Reynolds, J. Tennyson, J. Ladik, P. Pyykko, R. I. Maurer, Th. E. Simos, S. Wilson, S. E. O’Brien, D. Pugh, P. L. A. Popelier, A. J. Richardson, H. Stoll, M. Springborg, F. M. Aicken, Eds., Roy. Soc. Chem., Cambridge, 2000, pp. 143–198 (ISBN 978-0854042548)
J. R. Mohallem, Theor. Chim. Acta 107 (2002) 372 (https://doi.org/10.1007/s00214-002-0345-y)
R. F. W. Bader, Monatsh. Chem. 136 (2005) 819 (https://doi.org/10.1007/s00706-005-0307-x)
R. F. W. Bader, J. Phys. Chem., A 111 (2007) 7966 (https://doi.org/10.1021/jp073213k)
I. S. Bushmarinov, K. A. Lyssenko, M. Yu. Antipin, Russ. Chem. Rev. 78 (2009) 283 (https://doi.org/10.1070/RC2009v078n04ABEH004017)
Computational Chemistry Using the Quantum Theory of Atoms in Molecules (QTAIM), AIMAll Version 19.10.12, http://aim.tkgristmill.com (accessed 7th December 2023)
S. V. Volovik, G. G. Dyadyusha, V. I. Staninets, Regioselektivnost’ i reaktsionnaya sposobnost’ svobodnykh radikalov v protsessakh prisoedineniya i aromaticheskogo zameshcheniya, V. D. Pokhodenko, Ed., Naukova Dumka, Kiev, 1988 (ISBN: 5-12-000209-9)
S. V. Volovik, V. I. Staninets, N. S. Zefirov, Theor. Exp. Chem. 26 (1990) 390 (https://doi.org/10.1007/BF00530251)
S. V. Volovik, V. I. Staninets, N. S. Zefirov, Doklady Akad. Nauk Ros. 330 (1993) 321 (ISSN: 0869-5652)
V. S. Urusov, J. Struct. Chem. 35 (1994) 101 (https://doi.org/10.1007/BF02578507)
A. M. Rozen, B. V. Krupnov, Russ. Chem. Rev. 65 (1996) 973 (https://doi.org/10.1070/RC1996v065n11ABEH000241)
E. P. Buchikhin, A. M. Chekmarev, N. A. Bobyrenko, Russ. J. Inorg. Chem. 55 (2010) 790 (https://doi.org/10.1134/s0036023610050219)
Weitao Yang, W. J. Mortier, J. Amer. Chem. Soc. 108 (1986) 5708 (https://doi.org/10.1021/ja00279a008)
M. V. Lebedev, Semiconductors 35 (2001) 1291 (https://doi.org/10.1134/1.1418074)
P. W. Ayers, R. G. Parr, J. Amer. Chem. Soc. 122 (2000) 2010 (https://doi.org/10.1021/ja9924039)
F. Méndez, J. L. Gázquez, J. Amer. Chem. Soc. 116 (1994) 9298 (https://doi.org/10.1021/ja00099a054)
M. E. Kletskii, O. N. Burov, N. S. Fedik, S. V. Kurbatov, Chem. Heterocycl. Compounds 52 (2016) 700 (https://doi.org/10.1007/s10593-016-1952-1)
M. Head-Gordon, J. A. Pople, M. J. Frisch, Chem. Phys. Lett. 153 (1988) 503 (https://doi.org/10.1016/0009-2614(88)85250-3)
O. Christiansen, J. Chem. Phys. 119 (2003) 5773 (https://doi.org/10.1063/1.1601593)
M. Del Ben, J. Hutter, J. VandeVondele, J. Chem. Theory Comput. 8 (2012) 4177 (https://doi.org/10.1021/ct300531w)
M. Ochi, Sh. Tsuneyuki, Chem. Phys. Lett. 621 (2015) 177 (https://doi.org/10.1016/j.cplett.2015.01.009)
I. A. Abronin, G. M. Zhidomirov, Theor. Exp. Chem. 12 (1976) 68 (https://doi.org/10.1007/BF00524932)
G. M. Zhidomirov, A. A. Bagatur’yants, I. A. Abronin, Prikladnaya kvantovaya khimiya. Raschety reaktsionnoi sposobnosti i mekhanizmov khimicheskikh reaktsii, Khimiya, Moscow, 1979.