Synthesis and in silico ADMET evaluation of new thiazole and thiazolidine-4-one derivatives as non-ulcerogenic analgesic and anti-inflammatory agents Scientific paper
Main Article Content
Abstract
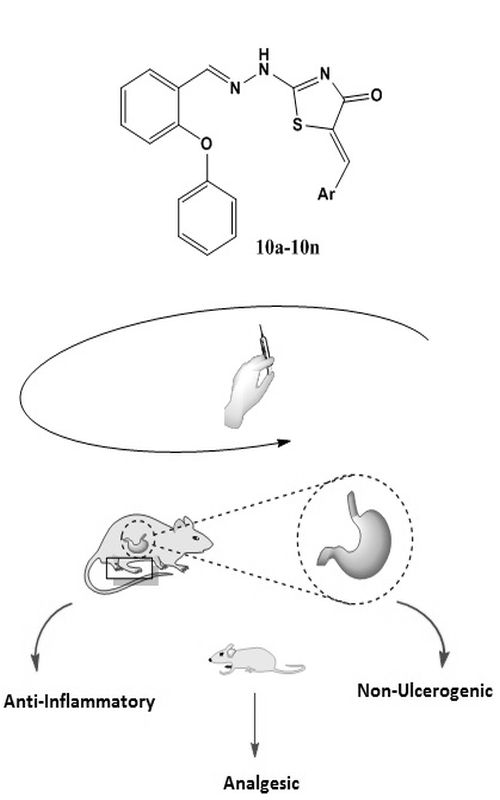
A series of 1,3-thiazoline-4-one derivatives bearing 2-phenoxyphenyl moiety, were synthesized as potential new analgesic and anti-inflammatory agents. The structures were confirmed by FT-IR, NMR and mass spectra. The abdominal constriction (writing) test was selected to evaluate analgesic activity and the results showed that nearly all of them were active, and the most potent was 10m with 96 % inhibition when compared to the control. The best compounds were selected to investigate the anti-inflammatory activity by carrageenan induced rat paw edema test. The results showed that compounds 8 and 10h were active from the 2nd to 5th hour of assessment. The ulcerogenic evaluation of two selected compounds showed that they are comparable with the vehicle control group and without any ulcerogenic effect potential. The target compounds showed an acceptable in silico ADME profile.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
A. Almasirad, M. Tajik, D. Bakhtiari, A. Shafiee, M. Abdollahi M, M. J. Zamani, R. Khorasani, H. Esmaily, J. Pharm. Pharma. Sci. 8 (2005) 419 (https://sites.ualberta.ca/~csps/JPPS8(3)/A.Shafiee/mefenamic.htm)
M. Shekarchi, L. navidpour, A. Rajabi-Khorami, M. Shekarchi, A. Partoazar, H. Shafaroodi, N. Rahmanipour, A. Shafiee, M. Shekarchi, Iran. J. Pharm. Res. 10 (2011) 369 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828903/)
S. Nikfar, M. Abdollahi, F. Etemad, M. Sharifzadeh, Gen. Pharmacol. 29 (1997) 583 (https://doi.org/10.1016/S0306-3623(96)00575-7)
C. Charlier, C. Michaux. Eur. J. Med. Chem. 38 (2003) 645 (https://doi.org/10.1016/S0223-5234(03)00115-6)
A. Almasirad, R. Hosseini, H. Jalalizadeh, Z. Rahimi-Moghaddam, N. Abaeian, M. Janafrooz, M. Abbaspour, V. Ziaee, A. Dalvandi, A. Shafiee, Biol. Pharma. Bull. 29 (2006) 1180 (https://doi.org/10.1248/bpb.29.1180)
A. Rineh, N. Mahmoodi, M. Abdollahi, A. Foroumadi, M. Sorkhi, A. Shafiee, Arch. Pharm. Chem. Life Sci. 340 (2007) 409 (https://doi.org/10.1002/ardp.200700045)
A. Shafiee, A. Rineh, A. Kebriaeezadeh, A. Foroumadi, V. Sheibani, M. R. Afarinesh, Med. Chem. Res. 18 (2009) 758 (https://doi.org/10.1007/s00044-009-9165-0)
D. H. Boschelli, D. T. Connor, D. A. Bornemeier, R. D. Dyer, J. A. Kennedy, P. J. Kuipers, G. C. Okonkwo, D. J. Schrier, C. D. Wright, J. Med. Chem. 36 (1993) 1802 (https://doi.org/10.1021/jm00065a002)
D.P. Wallach, V.R. Brown, Biochem. Biophys. Acta (BBA) – Lipids Lipid. Met. 663 (1981) 361 (https://doi.org/10.1016/0005-2760(81)90165-X)
A. Almasirad, A. Shafiee, M. Abdollahi, A. Neoparast, N. Shahrokhinejad, N. Vousooghi, S. A. Tabatabai, R. Khorasani, Med. Chem. Res. 20 (2011) 435 (https://doi.org/10.1007/s00044-010-9335-0)
A. Almasirad, S.A.Tabatabai, M. Faizi, A. Kebriaeezadeh, N. Mehrabi, A. Dalvandi, A. Shafiee. Bioorg. Med. Chem. Lett. 14 (2004) 6057 (https://doi.org/10.1016/j.bmcl.2004.09.072)
A. Almasirad, M. Sheikhha, R. Hosseini, S. A. Tabatabai, A. Shafiee, Arch. Pharm. 337 (2004) 193 (https://doi.org/10.1002/ardp.200300822)
A. Khomami, M. Rahimi, A. Tabei, P. Saniee, A. Mahboubi, A. Foroumadi, N. Nassiri-
-Koopaei, A. Almasirad, Mini. Rev. Med. Chem. 19 (2019) 239 (https://doi.org/10.2174/1389557518666181017142630)
S. Carradori, D. Secci, B. Bizzarri, P. Chimenti, C. De-Monte, P. Guglielmi, C. Campestre, D. Rivanera, C. Bordon, L. Jones-Brando, J. Enzyme Inhib. Med. Chem. 32 (2017) 746 (https://doi.org/10.1080/14756366.2017.1316494)
R. Bhutani, D. P. Pathak, G. Kapoor, A. Husain, M. A. Iqbal, Bioorg. Chem. 83 (2019) 6 (https://doi.org/10.1016/j.bioorg.2018.10.025)
N. U. Güzeldemirci, E. Pehlivan, L. Naesens, Marmara Pharm. J. 22 (2018) 237 (https://doi.org/10.12991/mpj.2018.61)
F. Yang, S. Peng, Y. Li, L. Su, Y. Peng, J. Wu, H. Chen, M. Liu, Z. Yi, Y. Chen, Org. Biomol. Chem. 14 (2016) 1727 (https://doi.org/10.1039/c5ob02250a)
M. Djukic, M. Fesatidou, I. Xenikakis, A. Geronikaki, V. T. Angelova, V. Savic, M. Pasic, B. Krilovic, D. Djukic, B. Gobeljic, M. Pavlica, A. Djuric, I. Stanojevic, D. Vojvodic, L. Saso. Chem. Biol. Interact. 286 (2018) 119 (https://doi.org/10.1016/j.cbi.2018.03.013)
M. Faizi, R. Jahani, S. A. Ebadi, S. A. Tabatabai, E. Rezaee, M. Lotfaliei, M. Amini, A. Almasirad, EXCLI J. 16 (2017) 52 (https://doi.org/10.17179/excli2016-692)
G. N. Tageldin, S. M. Fahmy, H. M. Ashour, M. A. Khalil, R. A. Nassra, I. M. Labouta, Bioorg. Chem. 80 (2018) 164 (https://doi.org/10.1016/j.bioorg.2018.06.013)
D. Kaminskyy D, A. Kryshchyshyn, R. Lesyk, Eur. J. Med. Chem. 140 (2017) 542 (https://doi.org/10.1016/j.ejmech.2017.09.031)
K. Oda, T. Hida, T. Sakata, M. Nagai, Y. Sugata, T. Masui, H. Nogusa, Org. Process. Res Dev. 12 (2008) 442 (https://doi.org/10.1021/op800008w)
A. Almasirad, M. Nassiri Koopaei, A. Shafiee, N. Nassiri-Koopaei, M. J. Assarzadeh, A. Tabei, M. Ghadimi, J. Pharm. Health. Sci. 1 (2013) 137 (https://sid.ir/paper/188761/en)
M. Nassiri Koopaei, M. J. Assarzadeh, A. Almasirad, S. F. Ghasemi-Niri, M. Amini, A. Kebriaeezadeh, N. Nassiri-Koopaei, M. Ghadimi, A. Tabei, Iran. J. Pharm. Res. 12 (2013) 721 (https://doi.org/10.22037/ijpr.2013.1366)
S. Bondock, W. Khalifa, A. A. Fadda, Eur. J. Med. Chem. 42 (2007) 948 (https://doi.org/10.1016/j.ejmech.2006.12.025)
A. Almasirad, N. Vousooghi, S. A. Tabatabai, A. Kebriaeezadeh, A. Shafiee, Acta Chim. Slov. 54 (2007) 317 (https://acta-arhiv.chem-soc.si/54/graph/acta-54(2)-GA.htm)
K. Omar, A. Geronikaki, P. Zoumpoulakis, C. Camoutsis, M. Soković, A. Ćirić, J. Glamočlija, Bioorg. Med. Chem. 18 (2010) 426 (https://doi.org/10.1016/j.bmc.2009.10.041)
M. H. Shih, F. Y. Ke, Bioorg. Med. Chem. 12 (2004) 4633 (https://doi.org/10.1016/j.bmc.2004.06.033)
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 42717 (https://doi.org/10.1038/srep42717)
Gu Y . Gu, Z. Yu, Y. Wang, L. Chen, C. Lou, C. Yang, W. Li, G. Liu, Y . Tang, Nucleic Acids Res. 22 (2024) gkae298 (https://doi.org/10.1093/nar/gkae298)
S. M. I. Badr, Turkish J. Chem. 35 (2011) 131 (https://doi.org/10.3906/kim-1001-473)
C. Anamaria, D. Leonte, L. Vlase, L. Csaba Bencze, S. Imre, G. Marc, B. Apan, C. Mogoșan, V. Zaharia, Molecules 23 (2018) 2425 (https://doi.org/10.3390/molecules23102425)
M. J. Assarzadeh, A. Almasirad, A. Shafiee, M. Nassiri Koopaei, M. Abdollahi, Med. Chem. Res. 23 (2014) 948 (https://doi.org/10.1007/s00044-013-0697-y).