Effect of silver nanoparticles in treating and healing of burn wound Scientific paper
Main Article Content
Abstract
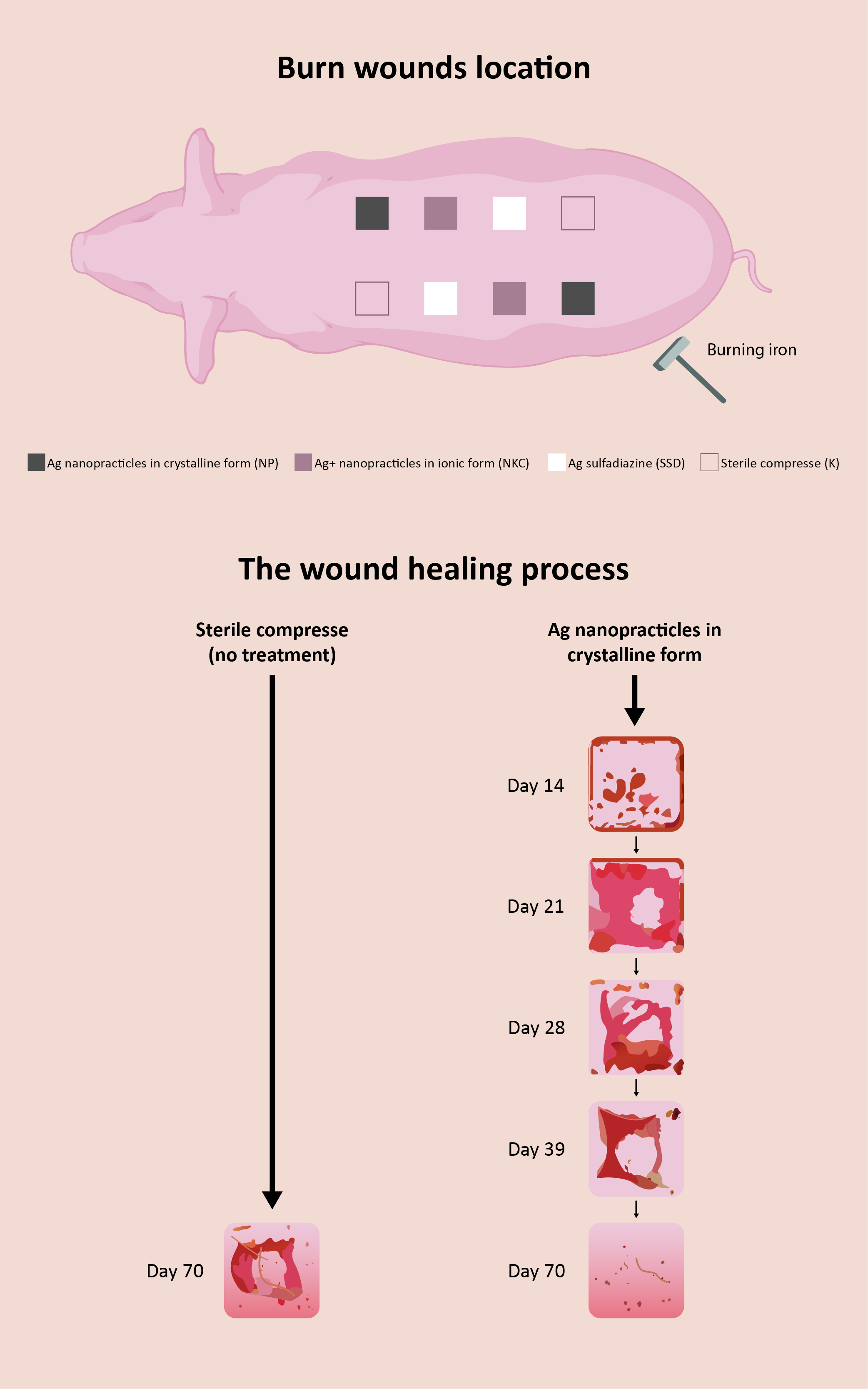
The paper investigates the effect of silver nanoparticles preparations on the rate of burn healing and scar quality. Three preparations for burn treatment were considered: one with silver sulfadiazine and two with silver nanoparticles woven into two types of dressing: one, of polyethylene and second, carboxymethyl cellulose. The experiment was performed on pigs, due to anatomical and pathophysiological similarities with human skin. All three silver preparations have antimicrobial properties with a beneficial effect on the healing of burns. Preparations with silver nanoparticles proved to be the most effective, since they encourage very fast burn epithelialization, affect reduction of the level of matrix metalloproteinase-9 in the environment of the burn wound, lead to faster expression of vascular endothetial growth factor – VEGF, cause less thickening of the epidermis and contractility, and improve tension characteristics of the scar compared to the preparation with silver sulfadiazine. By comparing results of healing parameters and evaluation of the scar achieved with preparations with silver nanoparticles, it was evident that the best overall results of local treatment were achieved with silver nanoparticles in crystalline form. Due to quantum-mechanics, surface and chemical oxidation–reduction (reactive oxygen species) phenomenological characteristics Ag nanoparticles in crystalline form have unique ability to catalyze rate of healing.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. H. Norahan, S. C. Pedroza-González, M. G. Sánchez-Salazar, M. M. Álvarez, G. T. de Santiago, Bioact. Mater. 24 (2023) 197 (https://doi.org/10.1016/j.bioactmat.2022.11.019)
M. J. Fresno, E. Selles, M. A. Camacho, Acta Technol. Legis Medicam 6 (1995) 220
M. C. Bonferoni, G. Sandri, S. Rossi, E. Dellera, A. Invernizzi, C. Boselli, B. Vigani, C. Caramella, F. Ferrari, A. I. Cornaglia, F. Riva, C. Del Fante, C. Perotti, Mar. Drugs 16 (2018) 56 (https://doi.org/10.3390/md16020056)
H. Maghsoudi, S. Monshidazadeh, M. Mrsgari. Ind. J. Surg. 73 (2011) 24 (https://doi.org/10.1007/s12262-010-0169-2)
H. M. Abdel-Mageed, N. Z. Abuelezz, R. A. Radwan, S. A. Mohamed, J. Microencapsul. 38 (2021) 414 (https://doi.org/10.1080/02652048.2021.1942275)
D. A. N. Moreno, M. S. Saladin, F. J. M. Viroel, M. M. J. Dini, T. B. Pickier, J. Amaral, C. A. dos Santos, V. M. Hanai-Yoshida, D. Grotto, M. Gerenutti, S. Hyslop, Y. Oshima-
-Franco, Adv. Pharm. Bull. 11 (2021) 130 (https://doi.org/10.34172/apb.2021.014)
J. M. Yang, Y. F. Huang, J. J. Dai, X. A. Shi, Y. Q. Zheng, Regen. Biomater. 8 (2021) rbab037 (https://doi.org/10.1093/rb/rbab037)
M. A. K. Ramadhan, A. N. Balasm, S. B. Kadhem, H. F. Al-Saedi, Maced. Vet. Rev. 44 (2021) 17 (https://doi.org/10.2478/macvetrev-2020-0032)
A. Kaler, A. K. Mittal, M. Katariya, H. Harde, A. K. Agrawal, S. Jain, U. C. Banarjee, J. Nanopart. Res. 16 (2014) 2605 (https://doi.org/10.1007/s11051-014-2605-x)
L. G. Wasef, H. M. Shaheen, Y. S. El-Sayed, T. I. A. Shalaby, D. H. Samak, M. E. Abd El-Hack, A. Al-Owaimer, I. M. Saadeldin, A. El-mleeh, H. Ba-Awadh, A. A. Swelum, Biol. Trace Elem. Res. 193 (2020) 456 (https://doi.org/10.1007/s12011-019-01729-z)
E. Sulastri, R. Lesmana, M. S. Zubair, A. F. A. Mohammed, K. M. Elamin, N. Wathoni, Heliyon 9 (2023) e18044 (https://doi.org/10.1016/j.heliyon.2023.e18044)
D. Olmos, G. M. Pontes-Quero, A. Corral, G. González-Gaitano, J. González-Benito, Nanomaterials 8 (2018) 60 (https://doi.org/10.3390/nano8020060)
M. Ashrafi, M. Hamadanian, A. R. Ghasemi, F. J. Kashi, Polym. Compos. 40 (2019) 3393 (https://doi.org/10.1002/pc.25200)
S. Dehghani, S. H. Peighambardoust, S. H. Fasihnia, S. J. Peighambardoust, N. K. Khosrowshahi, B. Gullón, J. M. Lorenzo, Food Anal. Methods 14 (2021) 98 (https://doi.org/10.1007/s12161-020-01856-7)
M. M. Mishyna, O. V. Hopta, G. O. Syrova, Y. A. Mozgova, S. G. Malanchuk, V. O. Makarov, V. L. Avramenko, I. A. Marchenko, O. S. Dubovyk, Y. M. Mishyn, J. Educ. Health Sport 11 (2021) 225 (https://doi.org/10.12775/JEHS.2021.11.01.022 )
S. Seino, Y. Ohkubo, T. Magara, H. Enomoto, E. Nakajima, T. Nishida, Y. Imoto, T. Nakagawa, Nanomaterials 12 (2022) 3046 (https://doi.org/10.3390/nano12173046)
T. Soltanolzakerin-Sorkhabi, M. Fallahi-Samberan, V. Kumaravel, Molecules 28 (2023) 5439 (https://doi.org/10.3390/molecules28145439)
S. Alven, S. A. Adeyemi, P. Ubanako, D. T. Ndinteh, Y. E. Choonara, B. A. Aderibigbe, Ther. Deliv. 14 (2023) 139 (https://doi.org/10.4155/tde-2022-0054)
Y. Cai, R. Gu, D., Yuhang, Z. Qi, Z. Ke, C. Cheng, H. Yang, J. Li, X. Yuan, J. Biomater. Appl. 38 (2023) 73 (https://doi.org/10.1177/08853282231173576)
C. Huang, M. Xiao, H. Cui, J. Wang, Y. Cai, Y. Ke, Int. J. Biol. Macromol. 252 (2023) 126495 (https://doi.org/10.1016/j.ijbiomac.2023.126495)
S. Perteghella, A. Garzoni, A. Invernizzi, M. Sorrenti, C. Boselli, A. Icaro Cornaglia, D. Dondi, S. Lazzaroni, G. Marrubini, C. Caramella, I. Catenacci, M. C. Bonferoni, Antioxidants 12 (2023) 273 (https://doi.org/10.3390/antiox12020273).