Study of the adsorption process between the phenolic compound catechin and the dietary fiber zymosan A: The influence of pH and concentration Scientific paper
Main Article Content
Abstract
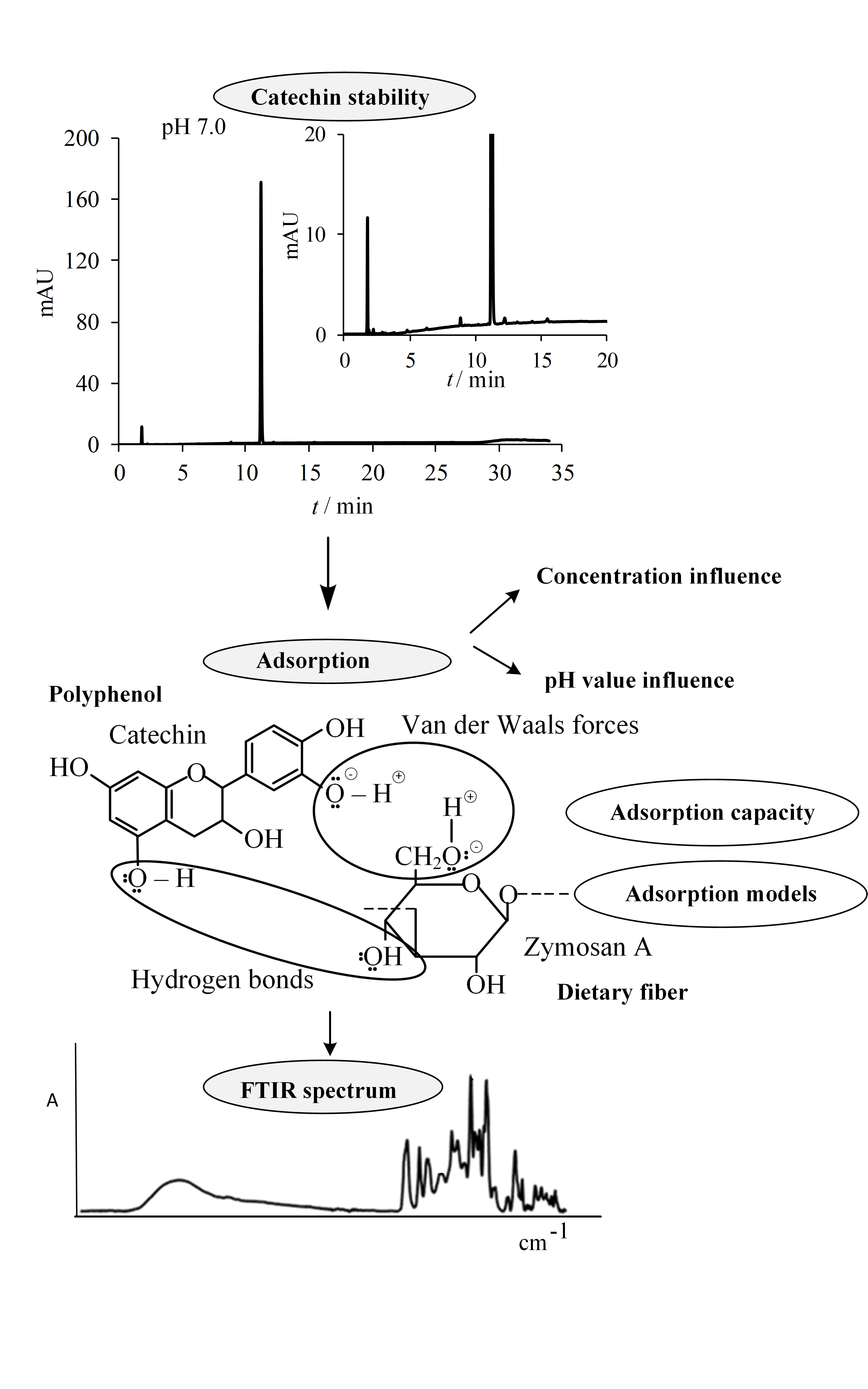
Polyphenolic compounds have shown various beneficial effects on human health as well as certain bioactivities such as interactions with dietary fiber. Factors that can influence their interactions with dietary fibers include the pH value, the polyphenolic compound concentration and compound stability. The aim of this work was to study the interactions between the polyphenolic compound catechin and the dietary fiber zymosan A from yeast through investigation of the adsorption process. The catechin stability and the influence of concentration and pH value on interactions were investigated. Catechin showed the lowest stability at pH 7.0 with degradation ratio from 6 to 15 %. The lowest adsorption capacity was at pH 7.0, then higher in water and the highest at pH 1.5. A Dubinin–Radushkevich adsorption model fit to the data and FTIR analysis indicates the presence of physical interactions between catechin and zymosan A. This study can contribute to better understanding of interactions of polyphenols and dietary fiber for possible design of functional food, or to increase bioaccessibility of polyphenols.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
F. Truzzi, C. Tibaldi, Y. Zhang, G. Dinelli, E. D’Amen, Int. J. Mol. Sci. 2 (2021) 5541 (https://doi.org/10.3390/ijms22115514)
L. Jakobek, P. Matić, Trends Food Sci. Technol. 83 (2019) 235 (https://doi.org/10.1016/j.tifs.2018.11.024)
S. Ho, Y. Y. Thoo, D. J. Young, L. F. Siow, Food Chem. 275 (2019) 594 (https://doi.org/10.1016/j.foodchem.2018.09.117)
Z. Y. Cai, X. M. Li, J. P. Liang, L. P. Xiang, K. R. Wang, Y. L. Shi, R. Yang, M. Shi, J. H. Ye, J. L. Lu, X. Q. Zheng, Y. R. Liang, Molecules 23 (2018) 2346 (https://doi.org/10.3390/molecules23092346)
V. K. Ananingshig, A. Sharma, W. Zhou, Food Res. Int. 50 (2013), 469 (https://doi.org/10.1016/j.foodres.2011.03.004)
A. P. Neilson, A. S. Hopf, B. R. Cooper, M. A. Pereira, J. A. Bomser, M. G. Ferruzzi, J. Agric. Food Chem. 55 (2007) 8941 (https://doi.org/10.1021/jf071645m)
Q. V. Vuong, C. E. Stathopoulos, M. H. Nguyen, J. B. Golding, P. D. Roach, Food Rev. Int. 27 (2011) 227 (https://doi.org/10.1080/87559129.2011.563397)
A. E. Quirós-Sauceda, H. Palafox-Carlos, S. G. Sáyago-Ayerdi, J. F. Ayala-Zavala, L. A. Bello-Perez, E. Álvarez-Parrilla, L. A. de la Rosa, F. A. González-Córdova, G. A. González-Aguilar, Food Funct. 5 (2014) 1063 (https://doi.org/10.1039/C4FO00073K)
G. Venkatachalam, A. Senthilkumar, M. Doble, ACS Omega 5 (2020), 15973 (https://doi.org/10.1021/acsomega.0c01243)
T. Miura, N. Ohno, N. N. Miura, Y. Adachi, S. Shimada, T. Yadomae, FEMS Microbiol. Immunol. 24 (1999) 131 (https://doi.org/10.1111/j.1574-695X.1999.tb01274.x)
M. Salgado, S. Rodríguez-Rojo, R. L. Reis, M. José Cocero, A. R. C. Duarte, J. Supercrit. Fluids 127 (2017) 158 (https://doi.org/10.1016/j.supflu.2017.04.006)
A. Yiannikouris, J. Francois, L. Poughon, C.-G. Dussap, G. Bertin, G. Jeminet, J.-P. Jouany, J. Agric. Food Chem. 52 (2004) 3666 (https://doi.org/10.1021/jf035127x)
A. Yiannikouris, G. Andre, A. Poughon, J. Francois, C.-G. Dussap, G. Jeminet, G. Bertin, J.-P. Jouany, Biomacromolecules 7 (2006) 1147, (https://doi.org/10.1021/bm050968t)
T.R. Falcão, C. A. O. Rodrigues, A. A. de Araújo, C. A. A. X. de Medeiros, L. A. L. Soares, M. R. A. Ferreira, R. C. Vasconcelos, R. F. de Araújo Júnior, M. L. D. de Sousa Lopes, G. C. B. Guerra, BMC Complement Altern. Med. 19 (2019) 1 (https://doi.org/10.1186/s12906-019-2454-3)
P. Matić, Š. Ukić, L. Jakobek, Chem. Biochem. Eng. Q. 35 (2021) 177 (https://doi.org/10.15255/CABEQ.2020.1902)
A. Siemińska-Kuczer, M. Szymańska-Chargot, A. Zdunek, Food Chem. 373 (2022) 131487 (https://doi.org/10.1016/j.foodchem.2021.131487)
Y. Liu, D. Yiang, L. Sanguansri, Y. Cai, X. Le, Food Res. Int. 112 (2018) 225 (https://doi.org/10.1016/j.foodres.2018.06.044)
Y. Liu, D. Yiang, L. Sanguansri, M. A. Augustin, Food Chem. 271 (2019) 733 (https://doi.org/10.1016/j.foodchem.2018.08.005)
N. Li, L. S. Taylor, M. G. Ferruzzi, L. J. Mauer, J. Agric. Food Chem. 60 (2012) 12531 (https://doi.org/10.1021/jf304116s)
Y. Narita, K. Inouye, J. Agric. Food Chem. 61 (2013) 966 (https://doi.org/10.1021/jf304105w)
M. Shi, Y. Nie, X. Q. Zheng, J. L. Lu, Y. R. Liang, J. H. Ye, Molecules 21 (2016) 1345 (https://doi.org/10.3390/molecules21101345)
Z. Xu, L. Wei, Z. Ge, W. Zhu, Eur. Food Res. Technol. 240 (2015) 707 (https://doi.org/10.1007/s00217-014-2375-9)
M. L. Soto, A. Moure, H. Domínguez, J. C. Parajó, J. Food Eng. 105 (2011) 1 (https://doi.org/10.1016/j.jfoodeng.2011.02.010)
R. Gao, H. Liu, Z. Peng, Z. Wu, Y. Wang, G. Zhao, Food Chem., B 132 (2012) 1936 (https://doi.org/10.1016/j.foodchem.2011.12.029)
Z. Wu, H. Li, J. Ming, G. Zhao, J. Agric. Food Chem. 59 (2011) 378 (https://doi.org/10.1021/jf103003q)
J. Herrero-Martínez, M. Sanmartin, M. Rosés, E. Bosch, C. Ràfols, Electrophor. 26 (2005) 1886 (https://doi.org/10.1002/elps.200410258)
T. Raab, D. Barron, F. A. Vera, V. Crespy, M. Oliveira, G. Williamson, J. Agric. Food Chem. 58 (2010) 2138 (https://doi.org/10.1021/jf9034095)
Y. Gao, R. Yiang, J. Qie, J. Chen, D. Xu, W. Liu, Q. Gao, Carbohydr. Polym., A 90 (2012) 1411 (https://doi.org/10.1016/j.carbpol.2012.05.096)
H. T. Simonsen, M. S. Nielsen, N. J. Christensen, U. Christensen, T. V. La Cour, M. S. Motawia, B. P. M. Jespersen, S. B. Engelsen, B. L. Møller, J. Agric. Food Chem. 57 (2009) 2056 (https://doi.org/10.1021/jf802057v)
M. Veverka, T. Dubaj, J. Gallovič, V. Jorík, E. Veverková, M. Mičušík, P. Šimon, J. Funct. Foods 8 (2014) 309 (https://doi.org/10.1016/j.jff.2014.03.032)
A. M. Mendoza-Wilson, D. Glossman-Mitnik, J. Mol. Struct. 761 (2006) 97 (https://doi.org/10.1016/j.theochem.2006.01.001)
M. Krysa, M. Szymańska-Chargot, A. Zdunek, Food Chem. 393 (2022) 133430 (https://doi.org/10.1016/j.foodchem.2022.133430)
A. Semalty, M. Semalty, D. Sing, M. S. M. Rawat, J. Incl. Phenom. Macrocycl. Chem. 67 (2010) 253 (https://doi.org/10.1007/s10847-009-9705-8).