The interaction between 4-oxothiazolidine-2-ylidene thioamides and iodine: a regioselective two-component 4-oxothiazolidine-2-ylidene thioamide to thiazolo[3,2-c]pyrimidine transformation mediated by iodine Survey
Main Article Content
Abstract
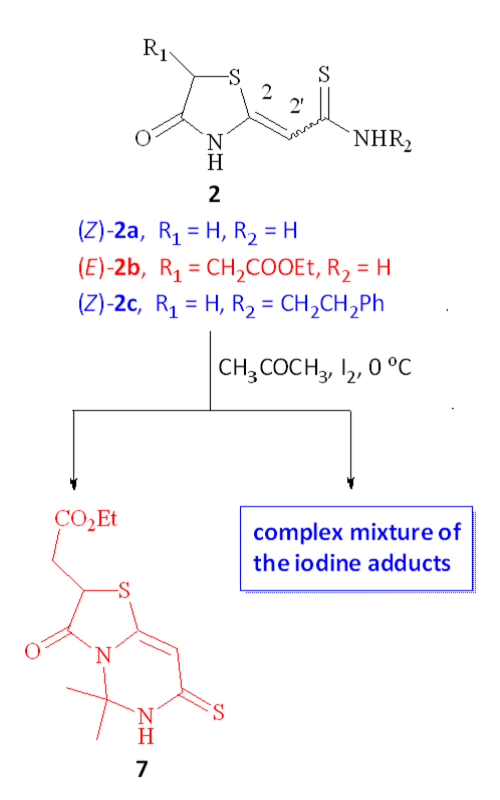
This study investigated the interaction between selected 4-oxothiazolidine-2-ylidene thioamides 2a-c and iodine in acetone. The interaction followed two main reaction pathways: (1) iodine-mediated cyclization resulting in the formation of thiazolopyrimidine 7, and (2) electrophilic iodine attack on the thioamide sulfur atom, producing a complex mixture of iodine adducts. Due to the equilibrium of Z/E isomerization being strongly shifted to the Z-isomer in polar solvents, only the thioamide (E)-2b successfully formed thiazolopyrimidine 7. The other two derivatives, (Z)-2a and (Z)-2c, followed the second reaction pathway. The factors influencing the heterocyclization of (E)-2b and its intermediates were thoroughly examined. This research provides the first description of an iodine-mediated heterocyclization leading to a thiazolopyrimidine scaffold. The literature on iodine-mediated heterocyclization leading to fused pyrimidines is limited, highlighting the significance of this study.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200026
References
A. Rašović, P. J. Steel, E. Kleinpeter, R. Marković, Tetrahedron 63 (2007) 1937 (https://doi.org/10.1016/j.tet.2006.12.075)
R. Marković, A. Rašović, 1,2-Dithioles, in Comprehensive Heterocyclic Chemistry III, A. R. Katritzky, Executive Ed., J. A. Joule, Vol. Ed., Elsevier Ltd., Oxford, UK, 4 (2008), p 893 (https://doi.org/10.1016/B978-008044992-0.00411-9)
A. Rašović, A. Koch, E. Kleinpeter, R. Marković, Tetrahedron 69 (2013) 10849 (https://doi.org/10.1016/j.tet.2013.10.088)
A. Rašović, 1,2- Dithioles, in Comprehensive Heterocyclic Chemistry IV, D. StC Black, J. Cossy, C. V. Stevens, Eds-in-Chief, R. A. Aitken, Vol. Ed, Elsevier Ltd., Oxford, UK, 4 (2022), p 766 (https://doi.org/10.1016/B978-0-12-818655-8.00137-2)
M. Stojanović, Z. Džambaski, B. Bondžić, J. Aleksić, M. Baranac-Stojanović, Curr. Org. Chem. 18 (2014) 1108 (http://dx.doi.org/10.2174/138527281809140624120436)
M. Gajić, Z. Džambaski, B. S. Ilić, G. Kocić, B. P. Bondžić, A. Šmelcerović, Chem. Biol. Interact. 345 (2021) 109536 (https://doi.org/10.1016/j.cbi.2021.109536)
J. Sandström, Top. Stereochem. 14 (1983) 83 (https://doi.org/10.1002/9780470147238.ch2)
E. Kleinpeter, J. Serb. Chem. Soc. 71 (2006) 1 (https://doi.org/10.2298/JSC0601001K)
M. Baranac-Stojanović, U. Klaumünzer, R. Marković; E. Kleinpeter, Tetrahedron 66 (2010) 8958 (https://doi.org/10.1016/j.tet.2010.09.040)
A. Rašović, V. Blagojević, M Baranac-Stojanović, E. Kleinpeter, R. Marković, D. M. Minić, New J. Chem. 40 (2016) 6364 (https://doi.org/10.1039/C6NJ00901H)
R. Marković, M. Baranac, N. Juranić, S. Macura, I. Cekić and D. Minić, J. Mol. Struct., 800 (2006), 85 (https://doi.org/10.1016/j.molstruc.2006.03.075)
R. Marković, A. Shirazi, Z. Džambaski, M. Baranac, D. Minić, J. Phys. Org. Chem. 17 (2004) 118 (https://doi.org/10.1002/poc.700)
P. T. Parvatkar, P. S. Parameswaran, S. G. Tilve, Chem. Eur. J. 18 (2012) 5460 (https://doi.org/10.1002/chem.201100324)
L.-Y. Zeng, C. Cai, J. Comb. Chem. 12 (2010) 35 (https://doi.org/10.1021/cc9000983)
M. Bakavoli, G. Bagherzadeh, M. Vaseghifar, A. Shiri, M. Pordel, M. Mashreghi, P. Pordeli, M. Araghi, Eur. J. Med. Chem. 45 (2010) 647 (https://doi.org/10.1016/j.ejmech.2009.10.051)
O. Foss, J. Johnsen, O. Tvedten, Acta Chem. Scand. 12 (1958) 1782 (https://doi.org/10.3891/acta.chem.scand.12-1782)
G. H.-Y. Lin, H. Hope, Acta. Cryst. B28 (1972) 643 (https://doi.org/10.1107/S0567740872002900)
A. J. Arduengo, E. M. Burgess, J. Am. Chem. Soc. 99 (1977) 2376 (https://doi.org/10.1021/ja00449a078)
C. Laurence, M. J. El Ghomari, J-Y. Le Questel, M. Berthelot, R. Mokhlisse, J. Chem. Soc., Perkin Trans. 2 7 (1998) 1545 (https://doi.org/10.1039/A803002B)
P. Deplano, J. R. Ferraro, M. L. Mercuri, E. F. Trogu, Coord. Chem. Rev. 188 (1999) 71 (https://doi.org/10.1016/S0010-8545(98)00238-0)
M. C. Aragoni, M. Arca, F. A. Devillanova, A. Garau, F. Isaia, V. Lippolis, G. Verani, Coord. Chem. Rev. 184 (1999) 271 (https://doi.org/10.1016/S0010-8545(98)00259-8)
P. D. Boyle, S. M. Godfrey, Coord. Chem. Rev. 223 (2001) 265 (https://doi.org/10.1016/S0010-8545(01)00386-1)
V. Daga, S. K. Hadjikakou; N. Hadjiliadis, M. Kubicki, J. H. Z. dos Santos, I. S. Butler, Eur. J. Inorg. Chem. 7 (2002) 1718 (https://doi.org/10.1002/1099-0682(200207)2002:7%3C1718::AID-EJIC1718%3E3.0.CO;2-S)
M. C. Aragoni, M. Arca, F. Demartin, F. A. Devillanova, A. Garau, F. Isaia, V. Lippolis, G. Verani, J. Am. Chem. Soc. 124 (2002) 4538 (https://doi.org/10.1021/ja012731k)
G. J. Corban, S. K. Hadjikakou, N. Hadjiliadis, M. Kubicki, E. R. T. Tiekink, I. S. Butler, E. Drougas, A. M. Kosmas, Inorg. Chem. 44 (2005) 8617 (https://doi.org/10.1021/ic0484396)
O. Hassel, Nobel Lecture, June 9 1970 „Structural Aspects of Interatomic Charge-Transfer Bonding“ (https://www.nobelprize.org/prizes/chemistry/1969/hassel/lecture/)
G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati, G. Terraneo, Chem. Rev. 116 (2016) 2478 (https://doi.org/10.1021/acs.chemrev.5b00484)
M. Breugst, D. von der Heiden, Chem. Eur. J. 24 (2018) 9187 (https://doi.org/10.1002/chem.201706136)
C. Laurence, J. Graton, M. Berthelot, M. J. El Ghomari, Chem. Eur. J. 17 (2011) 10431 (https://doi.org/10.1002/chem.201101071)
A. Jain, M. Gupta, A. Bhardwaj, T. R. Thapak, Res. J. Chem. Sci. 5 (2015) 39 (http://www.isca.me/rjcs/Archives/v5/i8/8.ISCA-RJCS-2015-112.php)
T. Steiner, Angew. Chem. Int. Ed. 41 (2002) 48 (https://doi.org/10.1002/1521-3773(20020104)41:1%3C48::AID-ANIE48%3E3.0.CO;2-U)
W. N. Speckamp, M. J. Moolenaar, Tetrahedron 56 (2000) 3817 (https://doi.org/10.1016/S0040-4020(00)00159-9)
J. Royer, M. Bonin, L. Micouin, Chem. Rev. 104 (2004) 2311 (https://doi.org/10.1021/cr020083x)
P. Wu, T. E. Nielsen, Chem. Rev. 117 (2017) 7811 (https://doi.org/10.1021/acs.chemrev.6b00806).