Investigating the therapeutic potential of monothiocarbohydrazones: A comprehensive in vitro evaluation of antioxidant, antimicrobial, and cytotoxic activities Scientific paper
Main Article Content
Abstract
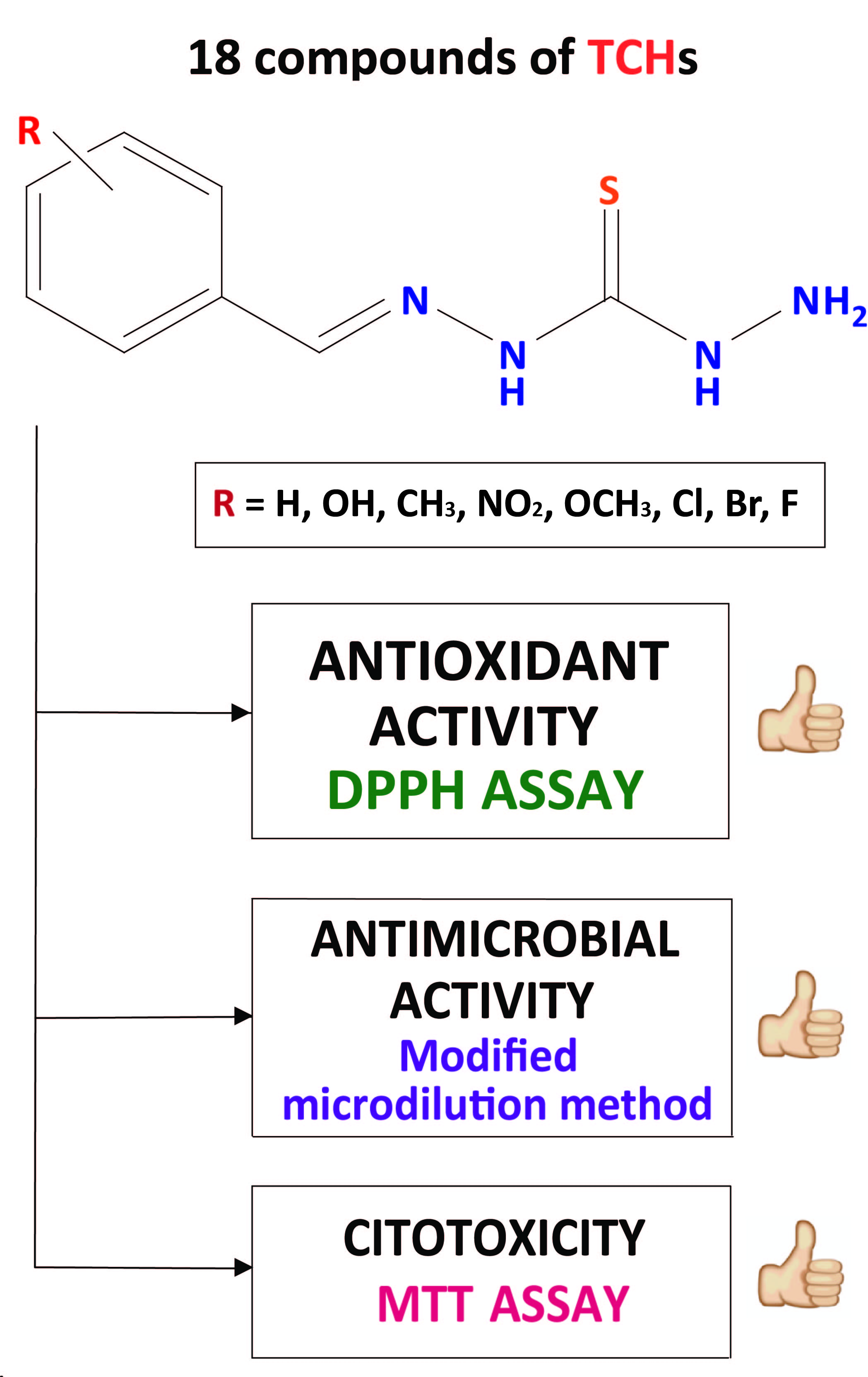
Organic compounds, particularly those with nitrogen and sulphur heteroatoms, constitute over 99 % of clinically approved drugs. Among these, thiocarbohydrazones have been extensively studied, with a focus on symmetrical bis-substituted compounds. However, asymmetric and monosubstituted thiocarbohydrazones remain underexplored, despite their demonstrated high biological potential. This study presents an in-depth in vitro evaluation of the antioxidant, antimicrobial, and cytotoxic properties of eighteen previously synthesized and characterized monothiocarbohydrazones. The antioxidant potential was assessed using the DPPH assay, while the antimicrobial activity was determined against Gram-positive bacteria using a modified broth microdilution susceptibility method. The cytotoxic effect was evaluated on human hepatocellular carcinoma using the colorimetric MTT assay. The results reveal that the investigated monothiocarbohydrazones exhibit significant antioxidant and antimicrobial activities. Furthermore, their activity and cytotoxicity are influenced by the stereochemistry of the molecule and the nature and position of the substituents. These findings provide valuable insights for future in vivo examinations and underscore the potential of monothiocarbohydrazones in drug development.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200125
References
P. C. Guha, S. C. Dey, J. Indian Chem. Soc. 2 (1925) 225 (https://ia902304.us.archive.org/18/items/sim_journal-of-the-indian-chemical-society_1925_2_index/sim_journal-of-the-indian-chemical-society_1925_2_index.pdf)
J. M. Cano Pavon, A. Garcia de Tores, C. Alcaraz, M. T. Siles Cordero, E. Vereda Alonso, Quimica Analitica. 13 (1994) 5
D. Rosales, A. G. Asuero, J. L. G. Ariza, The Analyst. 111 (1986) 449 (https://dx.doi.org/10.1039/AN9861100449)
S. P. Chaudhury, S. C. Shome, J. Indian. Chem. Soc. 68 (1991) 430 (https://zenodo.org/record/6160571/files/430-431.pdf)
R. B. Lucena, E. Morales, J. L. Gomez-Ariza, Farmaco. 49 (1994) 291 (https://pubmed.ncbi.nlm.nih.gov/8049011/)
R. B. Lucena, E. Morales, J. L. Gomez-Ariza, Farmaco. 49 (1994) 297 (https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=4270633)
S. B. Zanje, V. J. Suryavanshi, A. N. Kokare, A. A. Ghare, G. S. Kamble, P. N. Kamble, M. A. Anuse, J. Anal. Chem. 73 (2018) 438 (https://dx.doi.org/10.1134/S1061934818050131)
A. V. Sadlapurkar, U. B. Barache, A. B. Shaikh, S. H. Gaikwad, T. N. Lokhande, Int. J. Environ. An. Chem. 103 (2023) 3683 (https://dx.doi.org/10.1080/03067319.2021.1912333)
A. V. Sadlapurkar, U. B. Barache, A. B. Shaikh, P. C. Dhale, S. H. Gaikwad, T. N. Lokhande, Chem. Data Collect. 37 (2022) 100798 (https://dx.doi.org/10.1016/j.cdc.2021.100798)
G. Kiran, T. Maneshwar, Y. Rajeshwar, M. Sarangapani, J. Chem. 2013 (2013) 192039 (https://dx.doi.org/10.1155/2013/192039)
G. Mrđan, A. Tot, M. Vraneš, M. Rašeta, P. Knežević, T. Verbić, B. Matijević, B. Chem. Soc. Jpn. 95 (2022) 185 (https://dx.doi.org/10.1246/bcsj.20210326)
Y. Kaya, A. Ercag, A. Koca, J. Mol. Struct. 1102 (2015) 117 (https://dx.doi.org/10.1016/j.molstruc.2015.08.055)
Y. Kaya, A. Ercag, K. Kaya, J. Coord. Chem. 71 (2018) 3364 (https://dx.doi.org/10.1080/00958972.2018.1516872)
H. Muglu, M. S. Cavus, T. Bakir, H. Yakan, J. Mol. Struct. 1196 (2019) 819 (https://dx.doi.org/10.1016/j.molstruc.2019.07.002)
H. Yakan, T. K. Bakir, M. S. Cavus, H. Muglu, Res. Chem. Intermed. 46 (2020) 5417 (https://dx.doi.org/10.1007/s11164-020-04270-0)
T. K. Bakir, J. B. Lawag, Res. Chem. Intermed. 46 (2020) 2541 (https://dx.doi.org/10.1007/s11164-020-04105-y)
A. Bacchi, M. Carcelli, P. Pelagatti, C. Pelizzi, G. Pelizzi, F. Zani, J. Inorg. Biochem. 75 (1999) 123 (https://dx.doi.org/10.1016/S0162-0134(99)00045-8)
M. Shebl, S. M. E. Khalil, F. S. Al-Gohani, J. Mol. Struct. 980 (2010) 78 (https://dx.doi.org/10.1016/j.molstruc.2010.06.040)
M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary, Ni Nanting, M. I. Shaaban, M. M. El-Kerdawy, Synthetic Commun. 48 (2018) 2899
(https://dx.doi.org/10.1080/00397911.2018.1520889)
A. R. Božić, S. K. Bjelogrlić, I. T. Novaković, N. R. Filipović, P. M. Petrović, A. D. Marinković, T. R. Todorović, I. N. Cvijetić, Chemistryselect. 3 (2018) 2215
(https://dx.doi.org/10.1002/slct.201702691)
K. Gangarapu, S. Manda, A. Jallapally, S. Thota, S. K. Karki, J. Balzarini, E. De Clercq, H. Tokuda, Med. Chem. Res. 23 (2014) 1046 (https://dx.doi.org/10.1007/s00044-013-0684-3)
A. Božić, A. Marinković, S. Bjelogrlić, T. R. Todorović, I. N. Cvijetić, I. Novaković, C. D. Muller, N. R. Filipović, RSC Adv. 6 (2016) 104763 (https://doi.org/10.2298/JSC161220045B)
J. Wang, Y. T. Wang, Y. Fang, Y. L. Lu, M.X. Li, Toxicol. Res. 8 (2019) 862 (https://doi.org/10.1039/C9TX00109C)
M. H. Assaleh, S. K. Bjelogrlic, N. Prlainovic, I. Cvijetic, A. Bozic, I. Arandjelovic, D. Vukovic, A. Marinkovic, Arab. J. Chem. 15 (2022) 103532 (https://doi.org/10.1016/j.arabjc.2021.103532)
M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary, M. M. El-Kerdawy, Ni Nanting, Eur. J. Med. Chem. 128 (2017) 36 (https://doi.org/10.1016/j.ejmech.2017.01.030)
G. Mrdjan, Gy. Vastag, D. Škorić, M. Radanović, T. Verbić, M. Milčić, I. Stoiljković, O. Marković, B. Matijević, Struct. Chem. 32 (2021) 1231 (https://doi.org/10.1007/s11224-020-01700-y)
H. H. J. Gerets, K. Tilmant, B. Gerin, H. Chanteux, B. O. Depelchin, S. Dhalluin, F. A. Atienzar, Cell. Biol. Toxicol. 28 (2012) 69 (https://doi.org/10.1007/s10565-011-9208-4)
S. Yang, M. Ooka, R. J. Margolis, M. Xia, Cell Reports Methods 3 (2023) 100432 (https://doi.org/10.1016/j.crmeth.2023.100432)
C. J. Espin, G. Soler-Rivas, J. H. Wichers, J. Agr. Food Chem. 48 (2000) 648 (https://doi.org/10.1021/jf9908188)
I. Vulin, D. Tenji, I. Teodorovic, S. Kaisarevic, Chem-Biol. Interact. 365 (2022) 110082 (https://doi.org/10.1016/j.cbi.2022.110082)
J. L. Li, X.Y. Liu, J.T. Xie, Y.L. Di, F.X. Zhu, J. Phytopathol. 163 (2015) 239 (http://dx.doi.org/10.1111/jph.12312)
M. S. Cavus, H. Yakan, H. Muglu, T. Bakir, J. Phys. Chem. Solids. 140 (2020) 109362 (http://dx.doi.org/10.1016/j.jpcs.2020.109362)
M. Demurtas, A. Baldisserotto, I. Lampronti, D. Moi, G. Balboni, S. Pacifico, S. Vertuani, S. Manfredini, V. Onnis, Bioorg. Chem. 85 (2019) 568 (https://doi.org/10.1016/j.bioorg.2019.02.007)
D. Saxena, R. Maitra, R. Bormon, M. Czekanska, J. Meiers, A. Titz, S. Verma, S. Chopra, npj Antimicro. Resist. 1 (2023) 17 (https://doi.org/10.1038/s44259-023-00016-1)
R. Tamaian, A. Mot, R. Silaghi-Dumitrescu, I. Ionut, A. Stana, O. Oniga, C. Nastasa, D. Benedec, B. Tiperciuc, Molecules 20 (2015) 22188 (http://dx.doi.org/10.3390/molecules201219841)
J. L. Stigliani, V. Bernardes–Génisson, Ann. Pharm. Francaises 77 (2019) 126 (http://dx.doi.org/10.1016/j.pharma.2018.11.004)
R. K. C. De Paiva, J. F. Da Silva, H. A. Moreira, O. G. Pinto, L. T. F. M. Camargo, P. L. F. Naves, A. J Camargo, L. Ribeiro, L. M. Ramos, J. Brazil. Chem. Soc. 30 (2019) 164 (http://dx.doi.org/10.21577/0103-5053.20180167)
S. Podunavac–Kuzmanović, D. Cvetković, D. Barna, J. Serb. Chem. Soc. 73 (2008) 967 (http://dx.doi.org/10.2298/JSC0810967P)
M. B. Halli, R. B. Sumathi, Arab. J. Chem. 10 (2017) S1748 (https://doi.org/10.1016/j.arabjc.2013.06.025)
C. Bonaccorso, T. Marzo, D. La Mendola, Pharmaceuticals-Base. 13 (2020) 4 (http://dx.doi.org/10.3390/ph13010004)
S. M. Gomha, H. A. Abdelhady, D. Z. H. Hassain, A. H. Abdelmonsef, M. El-Naggar, M. M. Elaasser, H. K. Mahmoud, Drug Des. Dev. Ther. 15 (2021) 659 (http://dx.doi.org/10.2147/DDDT.S291579)
S. Bilginer, H. I. Gul, F. S. Erdal, H. Sakagami, S. Levent, I. Gulcin, C. T. Supuran, J. Enzym. Inhib. Med. Chem. 34 (2019) 1722 (http://dx.doi.org/10.1080/14756366.2019.1670657)
C. Bonaccorso, G. Grasso, N. Musso, V. Barresi, D. F. Condorelli, D. La Mendola, E. Rizzarelli, J. Inorg. Biochem. 182 (2018) 92 (http://dx.doi.org/10.1016/j.jinorgbio.2018.01.019)
Qurat-ul-Ain, M. Munira Taj, M. K. Khanlid, M. I. Choudhary, ArXiv. /abs/2310.00939 (2023) (https://doi.org/10.48550/arXiv.2310.00939).