Monte Carlo optimization-based QSAR modeling of Staphylococcus aureus inhibitory activity of coumarin-1,2,3-triazole hybrids Scientific paper
Main Article Content
Abstract
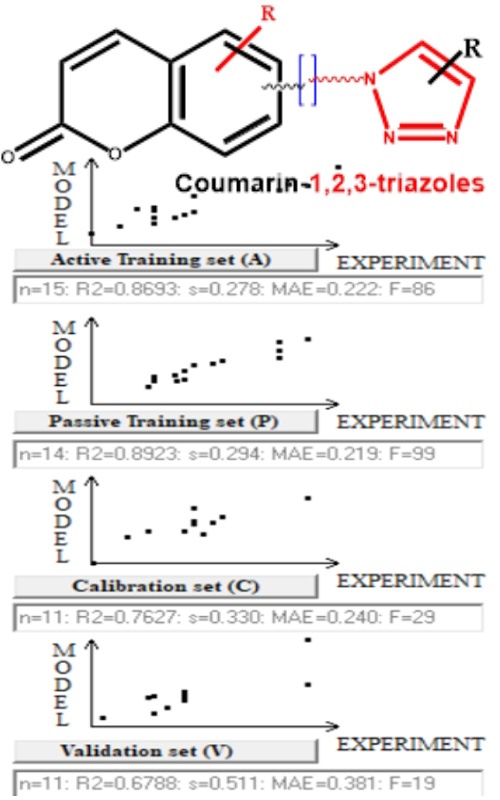
In this study, 51 coumarin-1,2,3-triazole hybrids with known minimum inhibitory concentration (MIC) values against Staphylococcus aureus were used for the generation of a Monte Carlo based optimized QSAR model on CORrelations And Logic (CORAL) software. The entire dataset was divided into four different sets, namely the training set (Tr), the invisible training set (iTr), the calibration set (C), and the validation set (V) of three random splits. For each split, five models were generated using various combinations of SMILES, graphs, and hybrid optimal descriptors with various connectivity indices. Finally, fifteen models were obtained from three random, non-identical splits. For the best model from each split, the correlation coefficient (r2) ranged from 0.9672 to 0.8693, while the cross-validated correlation coefficient (Q2) ranged from 0.9478 to 0.8250. The mean absolute error (MAE) for the best models was less than 0.065. Additionally, favorable values of the index of ideality of correlation (IIC) and correlation intensity index (CII) were reported, justifying the robustness, reliability, and predictive potential of the developed models. Further, good and bad fingerprints were estimated based on correlation weights for structural attributes.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Council of Science and Technology, U.P.
Grant numbers 3027
References
G. M. Cragg, D. J. Newman, Biochim. Biophys. Acta Gen. Subj. 1830 (2013) 3670 (https://doi.org/10.1016/j.bbagen.2013.02.008)
D. J. Newman, G. M. Cragg, J. Nat. Prod. 83 (2020) 770 (https://doi.org/10.1021/acs.jnatprod.9b01285)
H. C. Upadhyay, Lett. Drug Des. Discov. 20 (2023) 373 (https://doi.org/10.2174/157018082004230113144404)
H. C. Upadhyay, G. R. Dwivedi, M. P. Darokar, V. Chaturvedi, S. K. Srivastava, Planta Med. 78 (2012) 79 (https://doi.org/10.1055/s-0031-1280256)
M. Dickson, J. P. Gagnon, Discov. Med. 4 (2004) 172 (PMID: 20704981)
M. Dickson, J. P. Gagnon, Nat. Rev. Drug. Discov. 3 (2004) 417 (https://doi.org/10.1038/nrd1382)
H. C. Upadhyay, G. R. Dwivedi, S. Roy, A. Sharma, M. P. Darokar, S. K. Srivastava, ChemMedChem 9 (2014) 1860 (https://doi.org/10.1002/cmdc.201402027)
H. C. Upadhyay, B. S. Sisodia, H. S. Cheema, J. Agrawal, A. Pal, M. P. Darokar, S. K. Srivastava, Nat. Prod. Commun. 8 (2013) 1591 (https://doi.org/10.1177/1934578x1300801123)
H. C. Upadhyay, M. Singh, O. Prakash, F. Khan, S. K. Srivastava, D. U. Bawankule, SN Appl. Sci. 2 (2020) 2069 (https://doi.org/10.1007/s42452-020-03798-5)
M. Xiang, Y. Cao, W. Fan, L. Chen, Y. Mo, Comb. Chem. High Throughput Screen. 15 (2012) 328 (https://doi.org/10.2174/138620712799361825)
S. Surabhi, B. Singh, J. Drug Deliv. Therapeu. 8 (2018) 504 (https://doi.org/10.22270/jddt.v8i5.1894)
G. R. Dwivedi, H. C. Upadhyay, D. K. Yadav, V. Singh, S. K. Srivastava, F. Khan, N. S. Darmwal, M. P. Darokar, Chem. Biol. Drug. Des. 83 (2014) 482 (https://doi.org/10.1111/cbdd.12263)
M. Rudrapal, D. Chetia, J. Drug Deliv. Therapeu. 10 (2020) 225 (https://doi.org/10.22270/jddt.v10i4.4218)
P. Gramatica, QSAR Comb. Sci. 26 (2007) 694 (https://doi.org/10.1002/qsar.200610151)
OECD: OECD principles for the validation, for regulatory purposes, of (quantitative) structure-activity relationships models (2004)
E. Benfenati, A. A. Toropov, A. P. Toropova, A. Manganaro, R. Gonella Diaza, Chem. Biol. Drug. Des. 77 (2011) 471 (https://doi.org/10.1111/j.1747-0285.2011.01117.x)
A. T. K. Baidya, K. Ghosh, S. A. Amin, N. Adhikari, J. Nirmal, T. Jha, S. Gayen, New J. Chem. 44 (2020) 4129 (https://doi.org/10.1039/c9nj05825g)
K. Bagri, A. Kumar, M. Nimbhal, P. Kumar, Mol. Simul. 46 (2020) 777 (https://doi.org/10.1080/08927022.2020.1770753)
T. G. Kraljević, A. Harej, M. Sedić, S. K. Pavelić, V. Stepanić, D. Drenjančević, J. Talapko, S. Raić-Malić, Eur. J. Med. Chem. 124 (2016) 794 (https://doi.org/10.1016/j.ejmech.2016.08.062)
Y. L. Fan, X. Ke, M. Liu, J. Heterocycl. Chem. 55 (2018) 791 (https://doi.org/10.1002/jhet.3112)
H. C. Upadhyay, Curr. Top. Med. Chem. 21 (2021) 737 (https://doi.org/10.2174/1568026621666210303145759)
K. N. Mishra, H. C. Upadhyay, Frontiers Drug Discov. 2 (2022) 1072448 (https://doi.org/10.3389/fddsv.2022.1072448)
J. M. Madar, L. A. Shastri, S. L. Shastri, R. Guda, M. Holiyachi, N. S. Naik, S. Dodamani, S. Jalapure, V. A. Sungar, Chemical Data Collections 17–18 (2018) 219 (https://doi.org/10.1016/j.cdc.2018.09.005)
P. Yadav, B. Kumar, H. K. Gautam, S. K. Sharma, J. Chem. Sci. 129 (2017) 211 (https://doi.org/10.1007/s12039-016-1214-x)
S. Carmel Yesudass, P. Ranjan, H. P. Suresh, J. Heterocycl. Chem. 59 (2022) 309 (https://doi.org/10.1002/jhet.4385)
M. H. Shaikh, D. D. Subhedar, B. B. Shingate, F. A. Kalam Khan, J. N. Sangshetti, V. M. Khedkar, L. Nawale, D. Sarkar, G. R. Navale, S. S. Shinde, Med. Chem. Res. 25 (2016) 790 (https://doi.org/10.1007/s00044-016-1519-9)
S. M. Sutar, H. M. Savanur, C. Patil, G. M. Pawashe, G. Aridoss, K. M. Kim, R. G. Kalkhambkar, Chem. Data Coll. 28 (2020) 100480 (https://doi.org/10.1016/j.cdc.2020.100480)
A. V. Lipeeva, D. O. Zakharov, L. G. Burova, T. S. Frolova, D. S. Baev, I. V. Shirokikh, A. N. Evstropov, O. I. Sinitsyna, T. G. Tolsikova, E. E. Shults, Molecules 24 (2019) 2126 (https://doi.org/10.3390/molecules24112126)
M. N. Joy, Y. D. Bodke, S. Telkar, V. A. Bakulev, J. Mex. Chem. Soc. 64 (2020) 53 (https://doi.org/10.29356/jmcs.v64i1.1116)
X. M. Peng, K. V. Kumar, G. L. V. Damu, C. H. Zhou, Sci. China Chem. 59 (2016) 878 (https://doi.org/10.1007/s11426-015-0351-0)
P. Kumar, A. Kumar, J. Sindhu, SAR QSAR Environ. Res. 30 (2019) 63 (https://doi.org/10.1080/1062936X.2018.1564067)
A. A. Toropov, A. P. Toropova, Toxicol. Mech. Methods 29 (2019) 43 (https://doi.org/10.1080/15376516.2018.1506851)
A. A. Toropov, E. Benfenati, Curr. Drug. Discov. Technol. 4 (2007) 77 (https://doi.org/10.2174/157016307781483432)
A. Kumar, S. Chauhan, Arch. Pharm. (Weinheim) 350 (2017) e1600268 (https://doi.org/10.1002/ardp.201600268)
A. P. Toropova, A. A. Toropov, A. Roncaglioni, E. Benfenati, Molecules 28 (2023) 6587 (https://doi.org/10.3390/molecules28186587).