Phytochemical investigation from wood residues of Dalbergia spruceana Benth Short communication
Main Article Content
Abstract
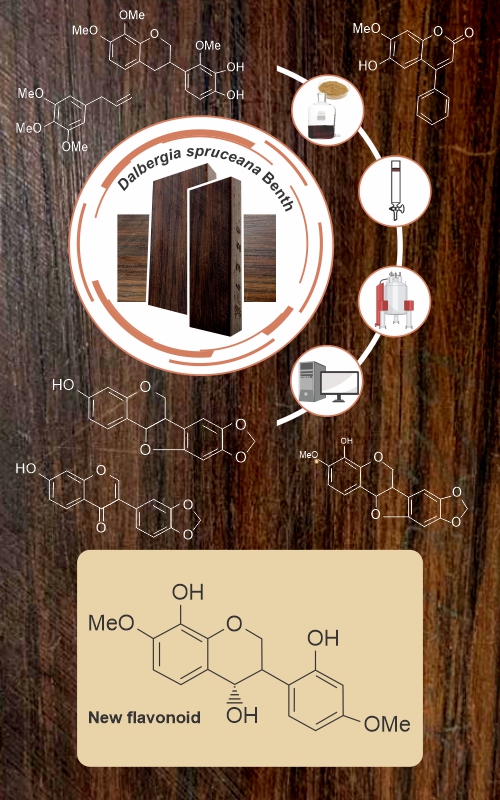
Dalbergia spruceana Benth (Fabaceae: Papilionoideae), known in the Brazilian Amazon as ¨jacarandá-do-pará” recognized for the natural resistance of its wood has little scientific information about its secondary metabolism. In this paper, we report a phytochemical study of the wood residues of D. spruceana using classical chromatographic techniques. Thus, the chromatographic fractionation of methanolic extract resulted in the isolation of phenylpropanoid, isoflavonoids of different types (pterocarpan, isoflavonol, isoflavan, isoflavone) in addition to a neoflavonoid. A new isoflavonoid with an oxygenation pattern not previously reported was elucidated as 8,4,2'-trihydroxy-7,4'-dimethoxyisoflavonol. The structures of all the isolated compounds were determined by using 1D- and 2D-NMR techniques, mass spectrometry ESI-MS and by comparison with literature data. Most of the compounds that were identified are isoflavonoids, which are types of flavonoids that are especially recognized for their contribution to the natural resistance of the wood.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Fundação de Amparo à Pesquisa do Estado do Amazonas
Grant numbers 01.02.016301.03412/2021-78
References
Tropicos – Missouri Botanical Garden 2024. Available from: https://www.tropicos.org/name/Search?name=Dalbergia%20spruceana (accessed on 06/05/2024)
L. A. G. Souza, Guia da biodiversidade de Fabaceae do Alto Rio Negro, INPA, Ed., Manaus-AM, 2012, p. 118 (ISBN 583.322)
J. R. V. Gama, J. C. Pinheiro, Floresta 40 (2010) 585 (ISSN 0015-3826).
A. A. Carvalho, L. R. Santos, J. S. Freitas, M. H. Chaves, Quim. Nova 9 (2020) 1294 (http://dx.doi.org/10.21577/0100-4042.20170588)
N-l. Zan, Z-H. Lu, X-Y. R-Y. Wang, N-Y. Liang, H-X. Huo, Y-F. Zhao, Y-L. Song, P-F. Tu, J. Zheng, J. Li, Nat. Prod. Res. 37 (2023) 928 (https://doi.org/10.1080/14786419.2022.2098494)
F. Shao, L. Panahipour, M. B, Sordi, F. Tang, R. Liu, R. Gruber, Molecules 27 (2022) 1321 (https://doi.org/10.3390/molecules27041321)
B. Lee, J.W. Lee, Q. Jin, D. S. Jang, S-J. Lee, D. Lee, J. T. Hong, Y. Kim, M. K. Lee, B. Y. Hwang, Bioorg. Med. Chem. Lett. 23 (2013) 4263 (https://doi.org/10.1016/j.bmcl.2013.04.032)
F. Shao, L. Panahipour, A. Omerbasic, F. Tang, R. Gruber, Clin. Oral Invest. 26 (2022) 5419 (https://doi.org/10.1007/s00784-022-04509-7)
S. Cheenpracha, T. Ritthiwigrom, C. Karalai, S. Laphookhieo, Phytochem. Lett. 5 (2012) 708 (https://doi.org/10.1016/j.phytol.2012.07.007)
H. Wang, W. Mei, Y. Zeng, W. Zuo, Z. Guo, L. Chen, H. Zhong, H. Dai, Phytochem. Lett. 9 (2014) 168 (https://doi.org/10.1016/j.phytol.2014.06.008)
F. R. Garcez, W. S. Garcez, L. Hamerski, C. H. Miguita, Quim. Nova 32 (2009) 407 (https://doi.org/10.1590/S0100-40422009000200026)
C. K. Swapan, L. Huang, F. Fekadu, D. M. Brown, M. C. Wani, M. E. Wall, J. C. Tucker, C. W. W. Beecher, A. D. Kinghorn, J. Nat. Prod. 58 (1995) 1966 (https://doi.org/10.1021/np50126a030)
H. J. Jung, S. S. Kang, S. K. Hyun, J. S. Choi, Arch. Pharm. Res. 28 (2005) 534 (https://doi.org/10.1007/BF02977754)
S. Cheenpracha, C. Karalai, C. Ponglimanont, A. Kanjana-Opas, J. Nat. Prod. 72 (2009) 1395 (https://doi.org/10.1021/np900077h)
N. T. Nhan, P. H. Nguyen, D. T. B. Hanh, D. T. T. My, N. P. D. Nguyen, T. B. Phong, D. C. To, Eur. J. Inflamm. 20 (2022) 172 (https://doi.org/10.1177/1721727X221132273)
N. Gampe, A. Darcsi, L. Kursinszki, S. Béni, J. Chromatogr., B 1091 (2018) 21 (https://doi.org/10.1016/j.jchromb.2018.05.023)
N. C. Veitch, Nat. Prod. Rep. 30 (2013) 988 (https://doi.org/10.1039/b511238a).