A simple method for identification of native collagen by reversed-polarity electrophoresis: Short report Short communication
Main Article Content
Abstract
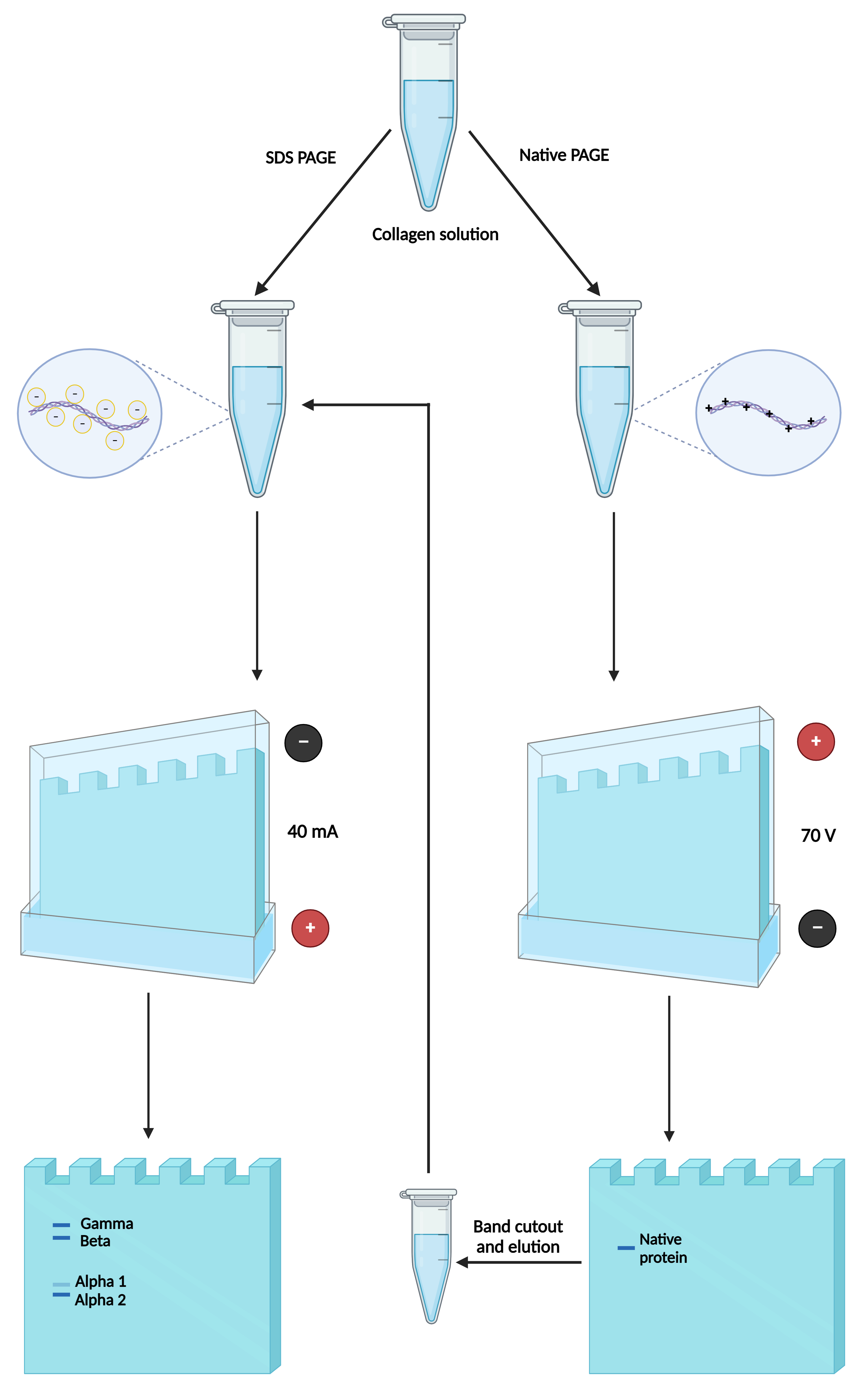
The high molecular weight of collagen and the high uncommon amino acid composition (proline and hydroxyproline) make the protein particular at structural and physicochemical levels compared to others. Polyacrylamide gel electrophoresis (PAGE) is a simple and inexpensive method to identify collagen integrity; however, native forms of proteins generally show low quality bands. In this work, we considered the charge of the protein to perform a very simple method to identify the native form of type I collagen, exhibiting an appropriate electrophoretic resolution. First, we determined the collagen charge at different pHs and then modified a previously published method by changing the gel buffer and reversing the polarity of the electrophoresis chamber by turning the power cords; now the protein was moved from the anode to the cathode. The result was well-resolved protein bands that maintained their classical structure without degradation after PAGE, which were confirmed by extracting the protein from the native-PAGE and electrophoresing it in a sodium dodecyl sulphate-PAGE. This advantage could be useful when the electrophoresed native collagen is used by Western blotting for recognition with antibodies.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. Ricard-Blum, Cold Spring Harb, Perspect. Biol. 3 (2011) a004978 (https://doi.org/10.1101%2Fcshperspect.a004978)
M. Shenoy, N. S. Abdul, Z. Qamar, B. M. A. Bahri, K. Z. K. Al Ghalayini, A. Kakti, Cureus 14 (2022) e24856 (https://doi.org/10.7759/cureus.24856)
I. N. Amirrah, Y. Lokanathan, I. Zulkiflee, M. F. M. R. Wee, A. Motta, M. B. Fauzi, Biomedicines 10 (2022) 2307 (https://doi.org/10.3390/biomedicines10092307)
Z. Deyl, M. Adam, J. Chrom. B: Biomed. Sci. App. 488 (1989) 161 (https://doi.org/10.1016/s0378-4347(00)82945-x)
J. R. Harris, A. Soliakov, R. J Lewis, Micron 49 (2013) 60 (https://doi.org/10.1016/j.micron.2013.03.004)
B. J. Bielajew, J. C. Hu, K. A. Athanasiou, Nat. Rev. Mater. 5 (2020) 730 (https://doi.org/10.1038/s41578-020-0213-1)
A. B. Nowakowski, W. J Wobig, D. H. Petering, Metallomics 6 (2014) 1068 (https://doi.org/10.1039/c4mt00033a)
S. Ricard-Blum, D. J. Hartmann, G. Ville, J. Chrom. B: Biomed. Sci. App. 530 (1990) 432 (https://doi.org/10.1016/s0378-4347(00)82346-4)
C. Arndt, S. Koristka, A. Feldmann, M. Bachmann, in: Electrophoretic Separation of Proteins. Methods in Molecular Biology, B. Kurien, R. Scofield, Eds., Humana Press, New York, 2019 (https://doi.org/10.1007/978-1-4939-8793-1_8)
U. K. Laemmli, Nature 227 (1970) 680 (https://doi.org/10.1038/227680a0)
J. A. Ramshaw, J. A Werkmeister, Anal. Biochem. 168 (1988) 82 (https://doi.org/10.1016/0003-2697(88)90013-9)
G. Leyva-Gómez, E. Lima, G. Krötzsch, R. Pacheco-Marín R. N. Rodríguez-Fuentes, D. Quintanar-Guerrero, E. Krötzsch, J. Phys. Chem., B 118 (2014) 9272 (https://doi.org/10.1021/jp502476x).
D. Shendi, J. Marzi, W. Linthicum, AJ. Rickards, DM. Dolivo, S. Keller, MA. Kauss, Q. Wen, TC. McDevitt, T. Dominko, K. Schenke-Layland, MW. Rolle. Acta Biomater. 100 (2019) 292 (https://doi.org/10.1016/j.actbio.2019.09.042).
L. Kumar, W. Colomb, J. Czerski, CR. Cox, SK. Sarkar. Protein Expr Purif. 148 (2018) 59 (https://doi.org/10.1016/j.pep.2018.04.001).
W. Kafienah, DJ. Buttle, D. Burnett, AP. Hollander. Biochem J. 330 (Pt 2) (1998) 897 (https://doi.org/10.1042/bj3300897).