Synthesis and mechanism of formation of hybrid structures comprising 2-oxochromene, thiazole and hydrazilidenechromene fragments Scientific paper
Main Article Content
Abstract
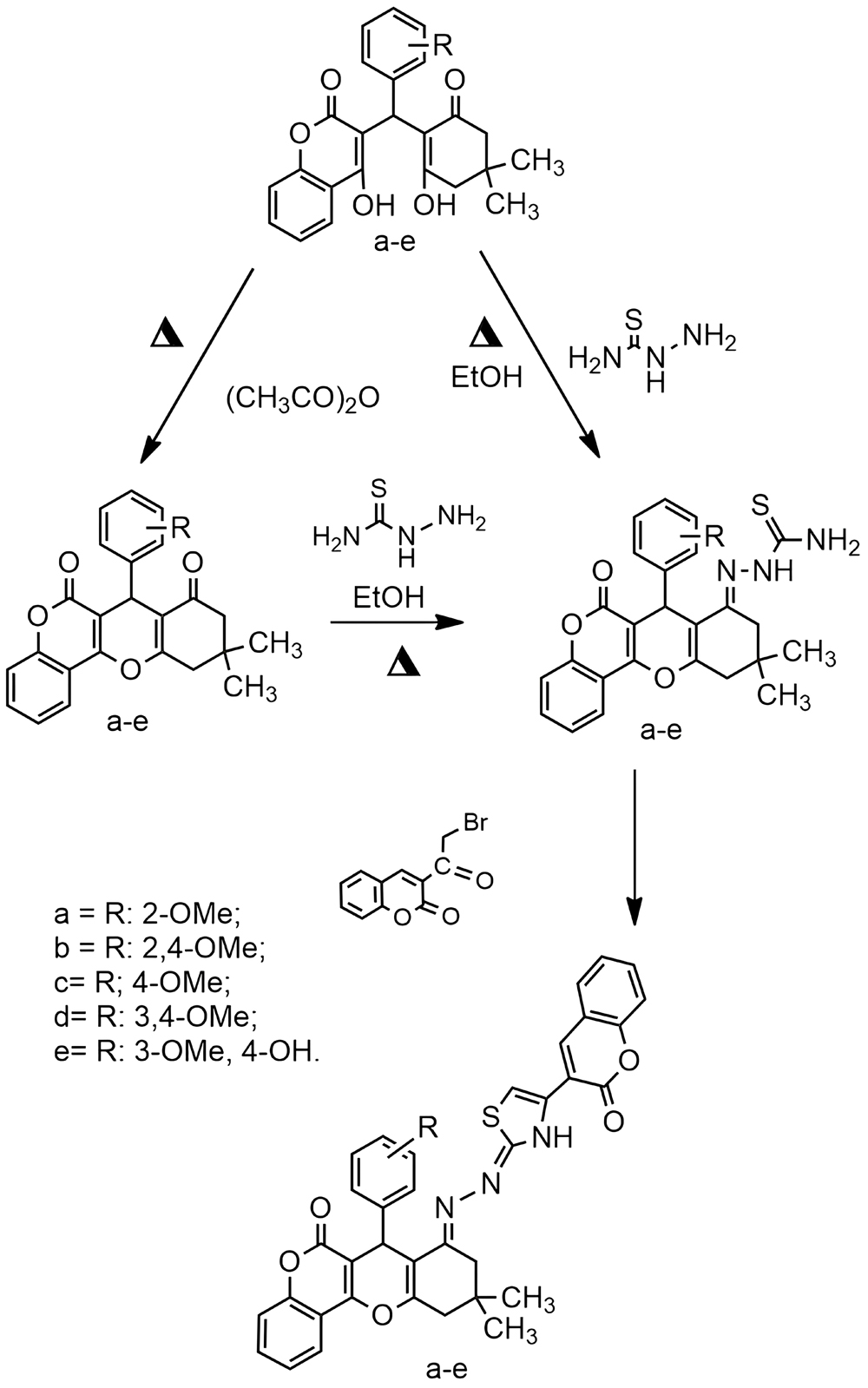
Molecules with a hybrid structure containing 1,3-, 1,5-dicarbonyl fragments, based on 2H-chromen-2-one, hold significant potential as biologically active substances. A direct method has been developed for the preparation of thiosemicarbazones 2-(7-(aryl)-10,10-dimethyl-6-oxo-7,9,10,11-tetrahydro-6H,8H-chromeno[4,3-b]chromen-8-ylidene)hydrazine-1-carbothioamides. Their further modification by reaction with 3-bromoacetyl-2H-chromen-2-one was carried out, involving the thioamide group to form hybrid structures comprising 2-oxochromene, thiazole and hydrazineylidenechromene fragments (yield 71–97%). It is shown that hydrazine-1-carbothioamides can be obtained from both the initial 1,5-dicarbonyl compound and the product of its intramolecular O-heterocyclization. A one-step method is preferable, because the one-step method is preferred over the more labour-intensive two-step approach (with a similar yield). A plausible reaction mechanism is presented, based on quantum chemical calculations of few possible tautomeric forms of the intermediates and the corresponding products. A comparative analysis of the 1H NMR spectrum of the experimental sample and the spectra of several possible final products calculated by a quantum chemical method has also confirmed the chosen reaction pathway.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28 (1993) 517 (https://doi.org/10.1016/0223-5234(93)90020-F)
C. Seoane, N. Martin, C. Pascual, L. L. Soto, Heterocycles 26 (1987) 2811 (https://doi.org/10.3987/R-1987-11-2811)
T. Pengsuparp, M. Serit, S. H. Hughes, D. D. Soejarto, J. M. Pezzuto, J. Nat. Prod. 59 (1996) 839 (https://doi.org/10.1021/np960399y)
B.-S. Yun, I.-K. Lee, I.-J. Ryoo, I.-D. Yoo, J. Nat. Prod. 64 (2001) 1238 (https://doi.org/10.1021/np0100946)
E.-W. Abd, H. F. Ashraf, Pharmaceuticals 5 (2012) 745 (https://doi.org/10.3390/ph5070745)
G. Rajitha, V. Ravibabu, G. Ramesh, B. Rajitha, R. Jobina, B. Siddhardha, V. Laxmi, Res. Chem. Intermed. 41 (2015) 9703 (https://doi.org/10.1007/s11164-015-1959-8)
S. B. Mohamed, Y. Rachedi, M. Hamdi, F. Le Bideau, C. Dejean, F. Dumas, Eur. J. Org. Chem. (2016) 2628 (https://doi.org/10.1002/ejoc.201600173)
R. Velpula, R. Deshineni, R. Gali, R. Bavantula, Res. Chem. Intermed. 42 (2016) 1729 (https://doi.org/10.1007/s11164-015-2114-2)
A. Yu. Kostritsky, A. Yu. Yegorova, O. V. Fedotova, Int. J. Appl. Fund. Res. 12 (2021) 88 (https://doi.org/10.17513/mjpfi.13336)
D. Armesto, W. M. Horspool, N. Martin, A. Ramos, C. Seoane, J. Org. Chem. 54 (1989) 3069 (https://doi.org/10.1021/jo00274a021)
P. Chellan, S. Nasser, L. Vivas, K. Chibale, G. S. Smith, J. Organomet. Chem. 695 (2010) 2225 (https://doi.org/10.1016/j.jorganchem.2010.06.010)
C. C. García, B. N. Brousse, M. J. Carlucci, A. G. Moglioni, M. M. Alho, G. Y. Moltrasio, N. B. D’Accorso, E. B. Damonte, Antivir. Chem. Chemother. 14 (2003) 61 (https://doi.org/10.1177/095632020301400205)
R. J. Glisoni, D. A. Chiappetta, L. M. Finkielsztein, A. G. Moglioni, A. Sosnik, New J. Chem. 34 (2010) 2047 (https://doi.org/10.1039/C0NJ00061B)
A. S. Shawali, J. Adv. Res. 5 (2014) 1 (https://doi.org/10.1016/j.jare.2013.01.004)
P. Zhan, X. Liu, Y. Cao, Y. Wang, C. Pannecouque, E. De Clercq, Bioorg. Med. Chem. Lett. 18 (2008) 5368 (https://doi.org/10.1016/j.bmcl.2008.09.055)
A. M. Vijesh, A. M. Isloor, S. Telkar, S. K. Peethambar, S. Rai, N. Isloor, Eur. J. Med. Chem. 46 (2011) 3531 (https://doi.org/10.1016/j.ejmech.2011.05.005)
A. D. Becke, J. Chem. Phys. 98 (1993) 5648 (https://doi.org/10.1063/1.464913)
A. D. Becke, Phys. Rev., A 38 (1988) 3098 (https://doi.org/10.1103/PhysRevA.38.3098)
C. Lee, W. Yang, R. G. Parr, Phys. Rev., B 37 (1988) 785 (https://doi.org/10.1103/PhysRevB.37.785)
R. Krishnan, J. S. Binkley, R. Seeger, J. A. Pople, J. Chem. Phys. 72 (1980) 650 (https://doi.org/10.1063/1.438955).