Conceptual DFT study based on the characterization of the local electrophilicity and nucleophilicity for intramolecular Diels–Alder reaction of the trans isomers of 4-[(4E)-4,6-heptadien-1-yl]-2-cyclohepten-1-one Scientific paper
Main Article Content
Abstract
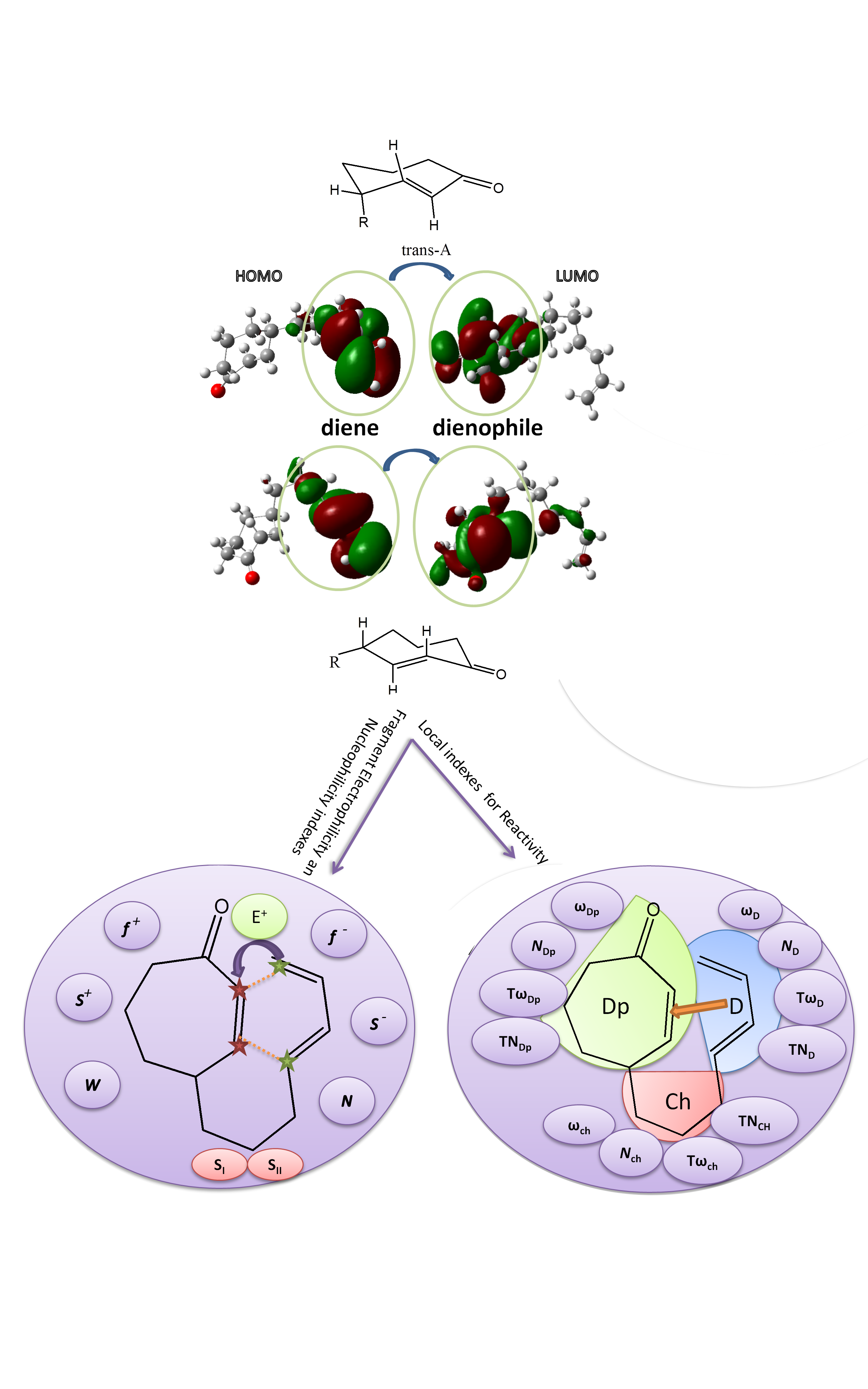
One of the most powerful methods for the rapid synthesis and formation of complex polycyclic molecules with biological interest involves the use of Diels–Alder (DA) reaction especially its intramolecular variant. The trans isomers of 4-substituted cycloheptenones were experimentally found to be excellent ethylenes, readily undergoing DA reactions. In this study we were interested to elucidate and predict the reactivity of the intramolecular DA (IMDA) reactions of the trans-A and trans-B isomers of 4-substituted cycloheptenone by means of the indexes of reactivity derived from DFT, at B3LYP/6-31G+(d,p) level of theory, using Gaussian09 program. In order to identify the reactional sites and to predict site selectivity of these compounds towards electrophilic and nucleophilic attack, the electrophilic 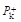 and nucleophilic
and nucleophilic 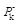 Parr functions, the local electrophilicity ωk and local nucleophilicity Nk were used in order to characterize the most electrophilic and nucleophilic sites. For the purpose, to make clear classification of the electrophilicity and nucleophilicity of the interacting diene and ethylene moieties within the same molecule, the local reactivity difference index Rk was used as a power full descriptor to study this IMDA cycloaddition. The fragments electrophilicity and nucleophilicity indices were calculated, according to the fragmentation model. The dual philicity index γ and the degree of transferability were determined. Here presented calculations showed, as expected, that the electronic transfer will take place from diene to ethylene moiety. The predictions thus made are in good agreement with other theoretical studies that analyse the electronic transfer through global electronic density transfer (GEDT).
Parr functions, the local electrophilicity ωk and local nucleophilicity Nk were used in order to characterize the most electrophilic and nucleophilic sites. For the purpose, to make clear classification of the electrophilicity and nucleophilicity of the interacting diene and ethylene moieties within the same molecule, the local reactivity difference index Rk was used as a power full descriptor to study this IMDA cycloaddition. The fragments electrophilicity and nucleophilicity indices were calculated, according to the fragmentation model. The dual philicity index γ and the degree of transferability were determined. Here presented calculations showed, as expected, that the electronic transfer will take place from diene to ethylene moiety. The predictions thus made are in good agreement with other theoretical studies that analyse the electronic transfer through global electronic density transfer (GEDT).
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
W. Carruthers, Cycloaddition reactions in organic synthesis, Elsevier, Amsterdam, 2013
R. B. Woodward, F. Sondheimer, D. Taub, K. Heusler, W. M. McLamore, J. Am. Chem. Soc. 74 (1952) 4223 (http://dx.doi.org/10.1021/ja01137a001)
C. P. Dell, J. Chem. Soc. Perkin I 22 (1998) 3873 (https://doi.org/10.1039/A803583K)
F. Fringuelli, A. Taticchi, E. Wenkert, Org. Prep. Proc. Int. 22 (1990) 131 (https://doi.org/10.1080/00304949009458194)
X. B. Xue, T. M. Lv, J. Y. Hou, D. Q. Li, X. X. Huang, S. J. Song, G. D. Yao, Phytomedicine 108 (2023) 154499 (https://doi.org/10.1016/j.phymed.2022.154499)
C. H. Mao, Q. M. Wang, R. Q. Huang, F. C. Bi, L. Chen, Y. X. Liu, J. Shang, J. Agric. Food Chem. 52 (2004) 6737 (https://doi.org/10.1021/jf048834e)
D. Caprioglio, S. Salamone, F. Pollastro, A. Minassi, Plants 10 (2021) 677 (https://doi.org/10.3390/plants10040677)
O. Acevedo, J. D. Evanseck, Org. Lett. 5 (2003) 649 (https://doi.org/10.1021/ol027408g)
E. J. Corey, Angew. Chem. Int. Ed. 41 (2002) 1650 (https://doi.org/10.1002/1521-3773(20020517)41:10%3C1650::AID-ANIE1650%3E3.0.CO;2-B )
S. Jin, P. Wessig, J. Liebscher, J. Org. Chem. 66 (2001) 3984 (https://doi.org/10.1021/jo0100897)
M. Juhl, D. Tanner, Chem. Soc. Rev. 38 (2009) 2983 (https://doi.org/10.1039/B816703F )
E. Ciganek, Org. React. 72 (2004) 1 (https://doi.org/10.1002/0471264180.or072.01)
H. V. Pham, R. S. Paton, A. G. Ross, S. J. Danishefsky, K. N. Houk, J. Am. Chem. Soc. 136 (2014) 2397 (https://doi.org/10.1021/ja410220w)
F. Fringuelli, M. Guo, L. Minuti, F. Pizzo, A. Taticchi, E. Wenkert, J. Org. Chem. 54 (1989) 710 (https://doi.org/10.1021/jo00264a039)
H. Dorr, V. H. Rawal, J. Am. Chem. Soc 121 (1999) 10229 (https://doi.org/10.1021/ja992287+)
T. J. Brocksom, J. Nakamura, M. L. Ferreira, U. Brocksom, J. Braz. Chem. Soc. 12 (2001) 597 (https://doi.org/10.1590/S0103-50532001000500004)
K. Yamamoto, I. Kawasaki, T. Kaneko, Tetrahedron Lett. 11 (1970) 4859 (https://doi.org/10.1016/S0040-4039(00)99728-4)
R. G. Parr, W. Yang, Ann. Rev. Phys. Chem. 46 (1995) 701 (https://doi.org/10.1146/annurev.pc.46.100195.003413)
L. R. Domingo, J. A. Sáez, Org. Biomol. Chem. 7 (2009) 3576 (https://doi.org/10.1039/B909611F)
P. K. Chattaraj, S. Duley, L. R. Domingo, Org. Biomol. Chem. 10 (2012) 2855 (https://doi.org/10.1039/C2OB06943A)
L. R. Domingo, P. Pérez, J. A. Sáez, RSC Adv. 3 (2013) 1486 (https://doi.org/10.1039/C2RA22886F)
L. R. Domingo, Molecules 21 (2016) 1319 (https://doi.org/10.3390/molecules21101319)
A. Benallou, H. E. A. El Abdallaoui, H. Garmes, Heliyon 4 (2018) e00504 (https://doi.org/10.1016/j.heliyon.2018.e00504 )
A. Benallou, H. Garmes, N. Knouzi, H. E. A. El Abdallaoui, Mor. J. Chem. 2 (2014) 110 (https://doi.org/10.48317/IMIST.PRSM/morjchem-v2i2.1912)
Gaussian 09, Gaussian, Inc., Wallingford, CT, 2009
L. R. Domingo, M. Ríos-Gutiérrez, P. Pérez, Molecules 25 (2020) 2535. (https://doi.org/10.3390/molecules25112535 )
W. Kohn, A. D. Becke, R. G. Parr, J Phys. Chem. 100 (1996) 12974 (https://doi.org/10.1021/jp960669l)
L. Meneses, W. Tiznado, R. Contreras, P. Fuentealba, Chem. Phys. Lett. 383 (2004) 181 (https://doi.org/10.1016/j.cplett.2003.11.019)
R. G. Parr, L. V. Szentpály, S. Liu, J. Am. Chem. Soc. 121 (1999) 1922 (https://doi.org/10.1021/ja983494x)
L. R. Domingo, P. Pérez, Org. Biomol. Chem. 9 (2011) 7168 (https://doi.org/10.1039/C1OB05856H)
P. K. Chattaraj, S. Duley, L. R. Domingo, Org. Biomol. Chem. 10 (2012) 2855 (https://doi.org/10.1039/C2OB06943A)
J. Soto-Delgado, L. R. Domingo, R. Contreras, Org. Biomol. Chem. 8 (2010) 3678 (https://doi.org/10.1039/C004628K)
L. R. Domingo, M. J. Aurell, P. Pérez, R. Contreras, Tetrahedron 58 (2002) 4417 (https://doi.org/10.1016/S0040-4020(02)00410-6)
L. R. Domingo, RSC Adv. 4 (2014) 32415 (https://doi.org/10.1039/C4RA04280H).