Evaluation of antimicrobial, anticancer and neuroprotective activities of silver nanoparticles (AgNPs) green-synthesized using a red pigment produced by Streptomyces sp. A23 strain isolated from Algerian bee pollen Scientific paper
Main Article Content
Abstract
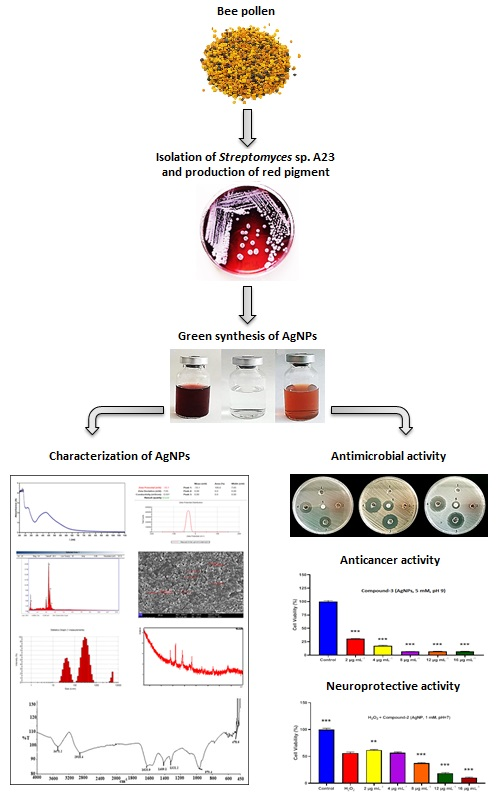
In this work, the red pigment of Streptomyces sp. A23 strain isolated from Algerian bee pollen was used for the green synthesis of silver nanoparticles (AgNPs) as well as for evaluating their antimicrobial, anticancer and neuroprotective activities. AgNPs were synthesized as a result of the reduction of 1 and 5 mM silver nitrate solutions at various pH values (5, 7 and 9) and were subsequently characterized. AgNPs (5 mM, pH 9) exhibited a maximum UV–Vis absorbance at 433 nm. Dynamic light scattering revealed that the average diameter was 112 nm. A zeta potential peak was found at –33 mV corresponding to the increased stability. XRD analysis confirmed the crystallization nature of the material. Furthermore, FT-IR analysis revealed the specific functional groups at 3471 to 478 cm-1. In addition, FE-SEM showed that the mean size of the spherical AgNPs was 54.5 nm in diameter. The presence of Ag was revealed by EDX analysis. Additionally, good antimicrobial activity was observed against Enterococcus faecalis ATCC 19433, Candida albicans ATCC 10231, Staphylococcus aureus ATCC 6538P, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 6633, Klebsiella pneumoniae ATCC 13883 and Escherichia coli ATCC 7839, with inhibition zones of 32, 30, 30, 27, 25, 20 and 19 mm, respectively. The lowest minimum inhibitory concentration and minimum bactericidal concentration were recorded against B. subtilis ATCC 6633, with a value of 62.5 μg mL-1. Intriguingly, all the synthesized AgNPs at concentrations of 2, 4 and 8 μg mL-1 had cytotoxic effects on SH-SY5Y neuroblastoma cell lines. In addition, AgNPs (1 mM, pH 7) exhibited the significant neuroprotective activity at the lowest tested concentration. Finally, the AgNPs synthesized using the red pigment of Streptomyces sp. strain A23 can be considered as promising therapeutic agents.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. Malik, K. Muhammad, Y. Waheed, Molecules 28 (2023) 6624 (https://doi.org/10.3390/molecules28186624)
L. Xu, Y. Y. Wang, J. Huang, C. Y. Chen, Z. X. Wang, H. Xie, Theranostics 10 (2020) 8996 (https://doi.org/10.7150/thno.45413)
A. Dhaka, S. C. Mali, S. Sharma, R. Trivedi, Results Chem. 6 (2023) 1 (https://doi.org/10.1016/j.rechem.2023.101108)
S. Barbuto Ferraiuolo, M. Cammarota, C. Schiraldi, O. F. Restaino, Appl. Microbiol. Biotechnol .105 (2021) 551 (https://doi.org/10.1007/s00253-020-11064-2)
A. Rosyidah, O. Weeranantanapan, N. Chudapongse, W. Limphirat, N. Nantapong, RSC Adv. 12 (2022) 4336 (https://doi.org/10.1039/D1RA08238H)
N. A. Al-Dhabi, A. K. M. Ghilan, M. V. Arasu, V. Duraipandiyan, J. Photochem. Photobiol., B 189 (2018) 176 (https://doi.org/10.1016/j.jphotobiol.2018.09.012)
S. S. Pallavi, H. A. Rudayni, A. Bepari, S. K. Niazi, S. Nayaka, Saudi J. Biol. Sci. 29 (2022) 228 (https://doi.org/10.1016/j.sjbs.2021.08.084)
N. Singh, B. Naik, V. Kumar, V. Kumar, S. Gupta, J. Microbiol. Biotech. Food Sci. 10 (2021) 604 (https://doi.org/10.15414/jmbfs.2021.10.4.604-608)
M. S. Mechouche, F. Merouane, C. E. H. Messaad, N. Golzadeh, Y. Vasseghian, M. Berkani, Environ. Res. 204 (2022) 1 (https://doi.org/10.1016/j.envres.2021.112360)
K. D. Datkhile, S. Chakraborty, P. P. Durgawale, S. R. Patil. Pharm. Nanotechnol. 12 (2024) 340 (https://doi.org/10.2174/2211738511666230913095001)
C. Ghosh, P. Sarkar, R. Issa, J. Haldar, Trends Microbiol. 27 (2019) 323 (https://doi.org/10.1016/j.tim.2018.12.010)
H. M. Abd-Elhady, M. A. Ashor, A. Hazem, F. M. Saleh, S. Selim, N. El Nahhas, S. H. Abdel-Hafez, S. Sayed, E. A. Hassan, Molecules 27 (2022) 1 (https://doi.org/10.3390/molecules27010212)
K. Aabed, A. E. Mohammed, Front. Bioeng. Biotechnol. 9 (2021) 1 (https://doi.org/10.3389/fbioe.2021.652362)
K. Kopeć, S. Szleszkowski, D. Koziorowski, S. Szlufik, Int. J. Mol. Sci. 24 (2023) 1 (https://doi.org/10.3390/ijms241210366)
M. Xu, X. Han, H. Xiong, Y. Gao, B. Xu, G. Zhu, J. Li, Molecules 28 (2023) 1 (https://doi.org/10.3390/molecules28135145)
F. E. Salem, H. M. Yehia, S. M. Korany, K. M. Alarjani, A. H. Al-Masoud, M. F. Elkhadragy, Food Sci. Technol. 42 (2022) 1 (https://doi.org/10.1590/fst.97322)
J. Baranwal, B. Barse, A. Di Petrillo, G. Gatto, L. Pilia, A. Kumar, Materials 16 (2023) 1 (https://doi.org/10.3390/ma16155354)
F. D. Zhu, Y. J. Hu, L. Yu, X. G. Zhou, J. M. Wu, Y. Tang, D. L. Qin, Q. Z. Fan, A. G. Wu, Front. Pharmacol. 12 (2021) 1 (https://doi.org/10.3389/fphar.2021.683935)
E. Hernández-Bolaños, D. Montesdeoca-Flores, E. Abreu-Yanes, M. L. Barrios, N. Abreu-Acosta, Curr. Microbiol. 77 (2020) 2510 (https://doi.org/10.1007/s00284-020-02030-2)
H. Mohamed, B. Miloud, F. Zohra, J. M. García-Arenzana, A. Veloso, S. Rodríguez-
-Couto, Int. J. Mol. Cell. Med. 6 (2017) 109 (https://doi.org/10.22088/acadpub.BUMS.6.2.5)
A. C. Smith, M. A. Hussey, ASM (2005) 1 (https://asm.org:443/Protocols/Gram-Stain-Protocols)
M. Lakhdar, D. Abir, M. Fatna, J. Agric. Appl. Bio. 4 (2023) 71 (https://doi.org/10.11594/jaab.04.01.08)
A. Tandale, M. Khandagale, R. Palaskar, S. Kulkarni, Int. J. Curr. Res. Life Sci. 7 (2018) 2397 (http://www.journalijcrls.com/sites/default/files/issues-pdf/01212.pdf)
E. T. Shirling, D. Gottlieb, Int. J. Syst. Evol. Microbiol. 16 (1966) 313 (https://doi.org/10.1099/00207713-16-3-313)
M. Mahfooz, S. Dwedi, A. Bhatt, S. Raghuvanshi, M. Bhatt, P. K. Agrawal, Int. J. Curr. Microbiol. App. Sci. 6 (2017) 4084 (https://doi.org/10.20546/ijcmas.2017.607.424)
M. Goodfellow, K. A. Peter, H. J. Busse, M. F. Trujillo, W. Ludwig, K. I. Suzuki, A. Parte, Bergey’s Manual of Systematic Bacteriology, Springer, New York, 2012 (https://doi.org/10.1007/978-0-387-68233-4)
F. Z. Djebbah, N. A. Al-Dhabi, M. V. Arasu, L. Belyagoubi, F. Kherbouche, D. E. Abdelouahid, B. Ravindran, J. King Saud Univ. Sci. 34 (2022) 101719 (https://doi.org/10.1016/j.jksus.2021.101719)
M. S. Almuhayawi, M. S. Mohamed, M. Abdel-Mawgoud, S. Selim, S. K. Al Jaouni, H. AbdElgawad, Biology 10 (2021) 1 (https://doi.org/10.3390/biology10030235)
T. Nuanjohn, N. Suphrom, N. Nakaew, W. Pathom-Aree, N. Pensupa, A. Siangsuepchart, J. Jumpathong, Molecules 28 (2023) 1 (https://doi.org/10.3390/molecules28165949)
N. K. Ahila, V. S. Ramkumar, S. Prakash, B. Manikandan, J. Ravindran, P. K. Dhanalakshmi, E. Kannapiran, Biomed. Pharmacother. 84 (2016) 60 (https://doi.org/10.1016/j.biopha.2016.09.004)
F. D. Koca, M. G. Halici, Y. Işik, G. Ünal, Inorg. Nano-Met. Chem. (2022) 1 (https://doi.org/10.1080/24701556.2022.2078351)
A. K. Shukla, S. Iravani, Green Synthesis, Characterization and Applications of Nanoparticles, Elsevier, Amsterdam, 2019 (https://doi.org/10.1016/C2017-0-02526-0)
M. Balouiri, M. Sadiki, S. K. Ibnsouda, J. Pharm. Anal. 6 (2016) 71 (https://doi.org/10.1016/j.jpha.2015.11.005)
M. Abou-Dobara, M. Mousa, M. Hasaneen, S. Nabih, J. Agric. Chem. Biotech. 9 (2018) 283 (https://doi.org/10.21608/jacb.2018.37039)
S. Jabeen, R. Qureshi, M. Munazir, M. Maqsood, M. Munir, S. H. Shah, B. Z. Rahim, Mater. Res. Express 8 (2021) 092001 (https://doi.org/10.1088/2053-1591/ac1de3)
I. Khan, K. Saeed, I. Khan, Arab. J. Chem. 12 (2019) 908 (https://doi.org/10.1016/j.arabjc.2017.05.011)
N. Bano, D. Iqbal, A. Al Othaim, M. Kamal, H. M. Albadrani, N. A. Algehainy, Roohi, Sci. Rep. 13 (2023) 4150 (https://doi.org/10.1038/s41598-023-30215-9)
K. Kamala, G. J. J. Kumar, D. Ganapathy, A. K. Sundramoorthy, P. Sivaperumal, Curr. Anal. Chem. 19 (2023) 550 (https://doi.org/10.2174/0115734110262574230927045451)
M. Asif, R. Yasmin, R. Asif, A. Ambreen, M. Mustafa, S. Umbreen, Dose-Response 20 (2022) 1 (https://doi.org/10.1177/15593258221088709)
S. Mourdikoudis, R. M. Pallares, N. T. Thanh, Nanoscale 10 (2018) 12871 (https://doi.org/10.1039/C8NR02278J)
T. Varadavenkatesan, R. Selvaraj, R. Vinayagam, Mater. Today: Proc. 23 (2020) 39 (https://doi.org/10.1016/j.matpr.2019.05.441)
M. Awashra, P. Młynarz, Nanoscale Adv. 5 (2023) 2674 (https://doi.org/10.1039/D2NA00534D)
Z. Jia, J. Li, L. Gao, D. Yang, A. Kanaev, Colloids Interfaces 7 (2023) 1 (https://doi.org/10.3390/colloids7010015)
Z. Gharari, P. Hanachi, H. Sadeghinia, T. R. Walker, Pharmaceuticals 16 (2023) 1 (https://doi.org/10.3390/ph16070992)
M. H. Moosavy, M. Guardia, A. Mokhtarzadeh, S. A. Khatibi, N. Hosseinzadeh, N. Hajipour, Sci. Rep. 13 (2023) 7230 (https://doi.org/10.1038/s41598-023-33632-y)
N. Kizildag, S. Cenkseven, F. D. Koca, H. Aka Saglıker, C. Darici, Eur. J. Soil Biol. 91 (2019) 18 (https://doi.org/10.1016/j.ejsobi.2019.01.001)
H. Fouad, G. Yang, A. A. El-Sayed, G. Mao, D. Khalafallah, M. Saad, H. Ga’al, E. Ibrahim, J. Mo, J. Nanobiotechnol. 19 (2021) 1 (https://doi.org/10.1186/s12951-021-01068-z)
I. A. M. Ali, A. B. Ahmed, H. I. Al-Ahmed, Sci. Rep. 13 (2023) 2256 (https://doi.org/10.1038/s41598-023-29412-3)
S. Korpayev, H. Hamrayev, N. Aničić, U. Gašić, G. Zengin, M. Agamyradov, G. Agamyradova, H. Rozyyev, G. Amanov, Biomass Convers. Biorefin. 14 (2024) 24715 (https://doi.org/10.1007/s13399-023-04648-1)
K. Kalwar, D. Shan, Micro Nano Lett. 13 (2018) 277 (https://doi.org/10.1049/mnl.2017.0648)
M. K. Rasmussen, J. N. Pedersen, R. Marie, Nat. Commun. 11 (2020) 1 (https://doi.org/10.1038/s41467-020-15889-3)
P. R. More, S. Pandit, A. D. Filippis, G. Franci, I. Mijakovic, M. Galdiero, Microorganisms 11 (2023) 1 (https://doi.org/10.3390/microorganisms11020369)
A. Menichetti, A. Mavridi-Printezi, D. Mordini, M. Montalti, J. Funct. Biomater. 14 (2023) 1 (https://doi.org/10.3390/jfb14050244)
T. C. Dakal, A. Kumar, R. S. Majumdar, V. Yadav, Front. Microbiol. 7 (2016) 1 (https://doi.org/10.3389/fmicb.2016.01831)
A. Roy, O. Bulut, S. Some, A. K. Mandal, M. D. Yilmaz, RSC Adv. 9 (2019) 2673 (https://doi.org/10.1039/C8RA08982E)
Y. Shkryl, T. Rusapetova, Y. Yugay, A. Egorova, V. Silant’ev, V. Grigorchuk, A. Karabtsov, Y. Timofeeva, E. Vasyutkina, O. Kudinova, V. Ivanov, V. Kumeiko, V. Bulgakov, Int. J. Mol. Sci. 22 (2021) 9305 (https://doi.org/10.3390/ijms22179305)
X. Zhai, S. Shan, J. Wan, H. Tian, J. Wang, L. Xin, Neurotox. Res. 40 (2022) 1369 (https://doi.org/10.1007/s12640-022-00570-y)
F. D. Koca, G. Ünal, M. G. Halici, J. Nano Res. 59 (2019) 15 (https://doi.org/10.4028/www.scientific.net/JNanoR.59.15)
M. I. Alkhalaf, R. H. Hussein, A. Hamza, Saudi J. Biol. Sci. 27 (2020) 2410 (https://doi.org/10.1016/j.sjbs.2020.05.005).