In silico molecular docking and ADMET prediction of Ginkgo biloba biflavonoids as dual inhibitors of human HMG-CoA reductase and α-amylase Scientific paper
Main Article Content
Abstract
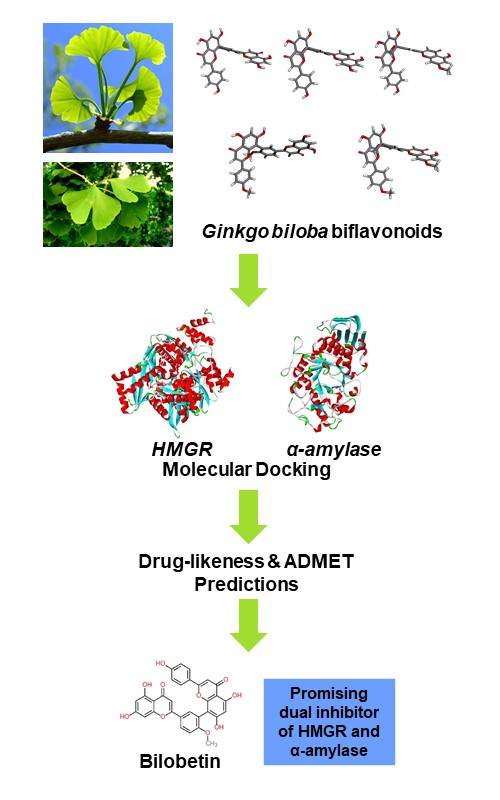
There is an increasing interest in investigating phytochemicals as inhibitors of HMG-CoA reductase (HMGR) and α-amylase enzymes. Inhibition of these enzymes helps manage hypercholesterolemia and diabetes by reducing cholesterol synthesis and blood sugar levels, respectively. In this study, computational techniques via molecular docking and ADMET prediction were used to determine the potential of five Ginkgo biloba biflavonoids (amentoflavone, bilobetin, ginkgetin, isoginkgetin and sciadopitysin) as dual inhibitors of HMGR and α-amylase. Amentoflavone (–42.26 kJ/mol) and bilobetin (–41.00 kJ/mol) exhibited stronger binding affinities to HMGR compared to the reference drug atorvastatin (–38.91 kJ/mol). For α-amylase, amentoflavone (–48.12 kJ/mol), bilobetin (–47.28 kJ/mol) and ginkgetin (–46.44 kJ/mol) exhibited stronger binding affinities compared to the reference drug acarbose (–43.93 kJ/mol). These docking results indicate that amentoflavone and bilobetin have the potential to act as dual inhibitors of these two enzymes. ADMET analysis showed that bilobetin demonstrated favorable oral bioavailability and drug-likeness, adhering to Lipinski’s rule of five. Despite exhibiting low gastrointestinal absorption, it was predicted to be neither mutagenic nor hepatotoxic. Therefore, bilobetin is a promising candidate for dual antidiabetic and antihypercholesterolemic applications. Further in vitro and in vivo studies are recommended to confirm these promising results.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. A. Friesen, V. W. Rodwell, Genome Biol. 5 (2004) 248 (https://doi.org/10.1186/gb-2004-5-11-248)
R. Jalaja, S. G. Leela, S. Mohan, M. S. Nair, R. K. Gopalan, S. B. Somappa, Bioorg. Chem. 108 (2021) 104664 (https://doi.org/10.1016/j.bioorg.2021.104664)
M. Son, A. Baek, S. Sakkiah, C. Park, S. John, K. W. Lee, PLoS ONE 8 (2013) e83496 (https://doi.org/10.1371/journal.pone.0083496)
N. Khatiwada, Z. Hong, Pharmaceutics 16 (2024) 214 (https://doi.org/10.3390/pharmaceutics16020214)
S. Ramkumar, A. Raghunath, S. Raghunath, Acta. Cardiol. Sin. 32 (2016) 631 (https://doi.org/10.6515/acs20160611a)
S. O. Oyedemi, B. O. Oyedemi, I. I. Ijeh, P. E. Ohanyerem, R. M. Coopoosamy, O. A. Aiyegoro, The Sci. World J. 2017 (2017) 3592491 (https://doi.org/10.1155/2017/3592491)
H. Kashtoh, K. Baek, Plants 12 (2023) 2944 (https://doi.org/10.3390/plants12162944)
O. A. Oluwagunwa, A. M. Alashi, R. E. Aluko, Front. Nutr. 8 (2021) 772903 (https://doi.org/10.3389/fnut.2021.772903)
S. Zhang, S. Zhu, M. N. Saqib, M. Yu, C. Du, D. Huang, Y. Li, Trends Food Sci. Technol. 143 (2024) 104303 (https://doi.org/10.1016/j.tifs.2023.104303)
M. Khalafi, A. A. Ravasi, A. Malandish, S. K. Rosenkranz, Diabetes Res. Clin. Pract. 186 (2022) 109815 (https://doi.org/10.1016/j.diabres.2022.109815)
A. Ceriello, Diabetes 54 (2005) 1 (https://doi.org/10.2337/diabetes.54.1.1)
J. S. Vidyashree, P. P. Shetti, S. C. Ghagane, Future J. Pharm. Sci. 10 (2024) 1 (https://doi.org/10.1186/s43094-024-00583-8)
U. Etxeberria, A. L. De La Garza, J. Campión, J. A. Martínez, F. I. Milagro, Expert Opin. Ther. Targets 16 (2012) 269 (https://doi.org/10.1517/14728222.2012.664134)
A. Mahdavi, M. Bagherniya, O. Fakheran, Ž. Reiner, S. Xu, A. Sahebkar, BioFactors 46 (2020) 906 (https://doi.org/10.1002/biof.1684)
A. P. Kalinovskii, O. V. Sintsova, I. N. Gladkikh, E. V. Leychenko, Int. J. Mol. Sci. 24 (2023) 16514 (https://doi.org/10.3390/ijms242216514)
K. Khadayat, B. P. Marasini, H. Gautam, S. Ghaju, N. Parajuli, Clin. Phytosci. 6 (2020) 1 (https://doi.org/10.1186/s40816-020-00179-8)
M. Akaberi, H. Baharara, M. S. Amiri, A. T. Moghadam, A. Sahebkar, S. A. Emami, Pharmacol. Res. – Mod. Chin. Med. 9 (2023) 100331 (https://doi.org/10.1016/j.prmcm.2023.100331)
D. Šamec, E. Karalija, S. Dahija, S. T. S. Hassan, Plants 11 (2022) 1381 (https://doi.org/10.3390/plants11101381)
V. D. Mouchlis, A. Afantitis, A. Serra, M. Fratello, A. G. Papadiamantis, V. Aidinis, I. Lynch, D. Greco, G. Melagraki, Int. J. Mol. Sci. 22 (2021) 1676 (https://doi.org/10.3390/ijms22041676)
D. R. Silva, J. De Cássia Orlandi Sardi, I. A. Freires, A. C. B. Silva, P. L. Rosalen, Eur. J. Pharmacol. 842 (2019) 64 (https://doi.org/10.1016/j.ejphar.2018.10.016)
T. T. Tuyen, P. C. Bach, H. V. Hai, P. T. H. Minh, N. T. H. Van, P. M. Quan, Vietnam J. Sci. Technol. 61 (2023) 355 (https://doi.org/10.15625/2525-2518/16945)
R. Maurus, A. Begum, L. K. Williams, J. R. Fredriksen, R. Zhang, S. G. Withers, G. D. Brayer, Biochemistry 47 (2008) 3332 (https://doi.org/10.1021/bi701652t)
E. S. Istvan, J. Deisenhofer, Science 292 (2001)1160 (https://doi.org/10.1126/science.1059344)
N. J. Agosto, P. B. Alambatin; J. Bacalso; J. Cabisada; B. D. Carating, Biol. Life Sci. Forum 35 (2024) 7 (https://doi.org/10.3390/blsf2024035007)
G. M. Morris, R. Huey, W. Lindstrom, M. F. Sanner, R. K. Belew, D. S. Goodsell, A. J. Olson, J. Comput. Chem. 30 (2009) 2785 (https://doi.org/10.1002/jcc.21256)
M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek, G. R. Hutchison, J. Cheminf. 4 (2012) 17 (https://doi.org/10.1186/1758-2946-4-17)
O. Trott, A. J. Olson, J. Comput. Chem. 31 (2009) 455 (https://doi.org/10.1002/jcc.21334)
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 42717 (https://doi.org/10.1038/srep42717)
D. E. V. Pires, T. L. Blundell, D. B. Ascher, J. Med. Chem. 58 (2015) 4066 (https://doi.org/10.1021/acs.jmedchem.5b00104)
R D. Ramírez, J. Caballero, Molecules 23 (2018) 1038 (https://doi.org/10.3390/molecules23051038)
P. L. Kastritis, A. M. J. J. Bonvin, J. R. Soc. Interface 10 (2013) 20120835 (https://doi.org/10.1098/rsif.2012.0835)
Q. Xue, X. Liu, P. Russell, J. Li, W. Pan, J. Fu, A. Zhang, Ecotoxicol. Environ. Saf. 233 (2022) 113323 (https://doi.org/10.1016/j.ecoenv.2022.113323)
X. Du, Y. Li, Y. Xia, S. Ai, J. Liang, P. Sang, X. Ji, S. Liu, Int. J. Mol. Sci. 17 (2016) 144 (https://doi.org/10.3390/ijms17020144)
R. Patil, S. Das, A. Stanley, L. Yadav, A. Sudhakar, A. K. Varma, PLoS ONE 5 (2010) e12029 (https://doi.org/10.1371/journal.pone.0012029)
C. A. Lipinski, F. Lombardo, B. W. Dominy, P. J. Feeney, Adv. Drug. Deliv. Rev. 46 (2001) 3 (https://doi.org/10.1016/s0169-409x(00)00129-0)
D. Li, L. Chen, Y. Li, S. Tian, H. Sun, T. Hou, Mol. Pharm. 11 (2014) 716 (https://doi.org/10.1021/mp400450m)
P. J. Ameji, A. Uzairu, G. A. Shallangwa, S. Uba, J. Taibah Univ. Med. Sci. 18 (2023) 1417 (https://doi.org/10.1016/j.jtumed.2023.05.021).