Electrochemical synthesis and anticancer inhibitory effect of copper(II)-diclofenac/decanoic acid complexes on MCF 7 breast cancer cells Scientific paper
Main Article Content
Abstract
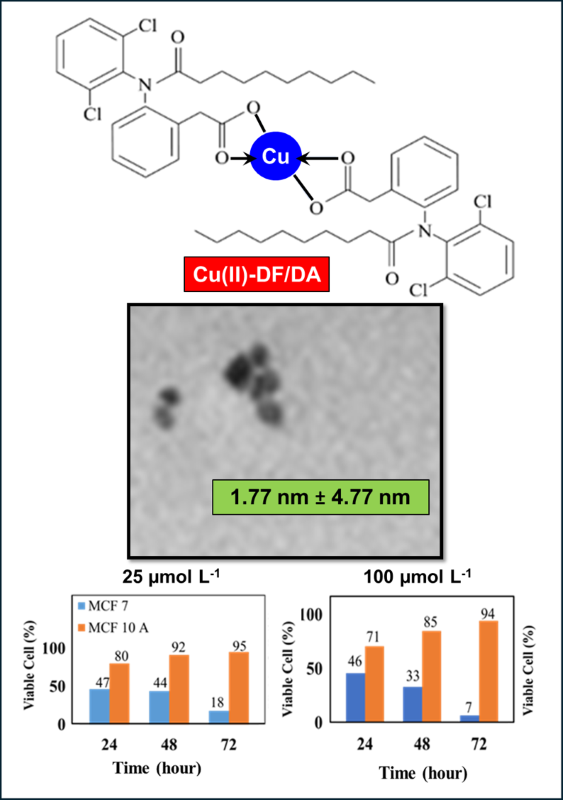
In this study, the Cu(II)-diclofenac/decanoic acid (Cu(II)-DF/DA) (copper(II) 2-[2-(2,6-dichloroanilino)phenyl]acetamide-decanoate) complex was synthesised using the electrochemical method by oxidising a Cu anode to release Cu2+ ions, with graphite and potassium nitrate (KNO3) serving as the cathode and supporting electrolyte, respectively. The synthesised Cu(II)-DF/DA complex underwent characterisation using ATR-FTIR, NMR, XRD and UV-Vis, confirming the success of the electrochemical synthesis. Surface morphology and particle size analyses using FESEM and TEM revealed that the synthesised Cu(II)-DF/DA complex possesses a thread-like structure with an average particle size of 4.77 nm ± 1.77 nm. Subsequently, the synthesised complex was used to assess the anticancer inhibitory effects on human breast cancer (MCF 7) and normal human breast epithelial (MCF 10A) cells. The treatment of MCF 7 cancer cells with Cu(II)-DF/DA at concentrations of 25 μmol L-1 and 100 μmol L-1 resulted in a significant reduction in cell viability, with only 18% and 7% of cells remaining viable after 72 hours, respectively. In contrast, nearly 90% of MCF 10A cells remained viable at comparable concentrations. This suggests that the synthesised Cu(II)-DF/DA shows potential as an effective and selective anticancer agent, being toxic to cancer cells while displaying lower toxicity to normal cells.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministry of Higher Education, Malaysia
Grant numbers FRGS/1/2020/STG04/USM/02/4
References
N. K. Singh, A. A. Kumbhar, Y. R. Pokharel, P. N. Yadav, J. Inorg. Biochem. 210 (2020) 111134 (https://doi.org/10.1016/j.jinorgbio.2020.111134)
A. K. Renfrew, Metallomics 6(8) (2014) 1324 (https://doi.org/10.1039/C4MT00069B)
N. Stevanović, M. Jevtović, D. Mitić, I. Z. Matić, M. D. Crnogorac, M. Vujčić, D. Sladić, B. Čobeljić, K. Anđelković, J. Serb. Chem. Soc. 87 (2022) 181 (https://doi.org/10.2298/JSC211203114S)
A. Alshargabi, J. Drug Deliv. Technol. 95 (2024) 105544 (https://doi.org/10.1016/j.jddst.2024.105544)
S. Choi, S. Kim, J. Park, S. E. Lee, C. Kim, D. Kang, Antioxidants 11 (2022) 1009 (https://doi.org/10.3390/antiox11051009)
L. Marinov, A. Georgiva, Y. Voynikov, R. Toshkova, I. Nikolova, M. Malchev, Biotechnol. Biotechnol. Equip. 35(1) (2021) 1118 (https://doi.org/10.1080/13102818.2021.1953401)
R. A. Poku, K. J. Jones, M. Van Baren, J. K. Alan, F. Amissah, Cancers (Basel) 12 (2020) 2683 (https://doi.org/10.3390/cancers12092683)
U. N. Das, J. Adv. Res. 11 (2018) 57 (https://doi.org/10.1016/j.jare.2018.01.001)
Y. Xu, S. Y. Qian, Biomed. J. 37(3) (2014) 112 (https://doi.org/10.4103/2319-4170.131378)
A. Guimarães, A. Venâncio, Toxins 14(3) (2022) 188 (https://doi.org/10.3390/toxins14030188)
M. Uchiyama, M. Oguri, E. H. Mojumdar, G. S. Gooris, J. A. Bouwstra, Biochim. Biophys. Acta 1858(9) (2016) 2050 (https://doi.org/10.1016/j.bbamem.2016.06.001)
M. Jóźwiak, A. Filipowska, F. Fiorino, M. Struga, Eur. J. Pharmacol. 871 (2020) 17293720 (https://doi.org/10.1016/j.ejphar.2020.172937)
A. Chrzanowska, P. Roszkowski, A. Bielenica, W. Olejarz, K. Stępień, M. Struga, Eur. J. Med. Chem. 185 (2020) 111810. (https://doi.org/10.1016/j.ejmech.2019.111810)
A. Narayanan, S. A. Baskaran, M. A. R. Amalaradjou, K. Venkitanarayanan, Int. J. Mol. Sci. 16(3) (2015) 5014 (https://doi.org/10.3390/ijms16035014)
N. Nordin, W. Z. Samad, E. Kardia, B. H. Yahaya, Nano 13(5) (2018) 1 (https://doi.org/10.1142/S1793292018500480)
R. P. Swain, R. Nagamani, S. Panda, J. Appl. Pharm. Sci. 5(07) (2015) 094 (https://doi.org/10.7324/JAPS.2015.50715)
P. B. Aielo, F. A. Borges, K. M. Romeira, M. C. R. Miranda, Mater. Res. 17(Suppl. 1) (2014) 146 (https://doi.org/10.1590/S1516-14392014005000010)
N. Nordin, B. H. Yahaya, M. R. Yusop, New J. Chem. 42(18) (2018) 15127 (https://doi.org/10.1039/C8NJ02783H)
A. Yoko, G.Y. Seong, T. Tomai, T. Adschiri, KONA Powder Part. J. 37 (2020) 28 (https://doi.org/10.14356/kona.2020002)
R. Suhara, M. Yamagami, H. Kamitakahara, A. Yoshinaga, Y. Tanaka, T. Takano, Cellulose 26 (2019) 355 (https://doi.org/10.1007/s10570-018-2027-5)
E. Moctezuma, E. Leyva, C. Lara‑Pérez, S. Noriega, A. Martínez‑Richa, Top. Catal. 63 (2020) 601 (https://doi.org/10.1007/s11244-020-01262-7)
G. D. Santos Souza, A. M. Amado, A. M. R. Teixeira, P. T. C. Freire, G. D. Saraiva, G. S. Pinheiro, S. G. C. Moreira, F. F. De Sousa, C. E. S. Nogueira, Cryst. Growth Des. 20 (2020) 281 (https://doi.org/10.1021/acs.cgd.9b01164)
L. S. Tan, H. L. Tan, K. Deekonda, Y. Y. Wong, S. Muniyandy, K. Hashim, J. Pushpamalar, Carbohydr. Polym. Technol. Appl. 2 (2021) 100084 (https://doi.org/10.1016/j.carpta.2021.100084)
Y. M. Long, Q. L. Zhao, Z. L. Zhang, Z. Q. Tian, D. W. Pang, Analyst 137 (2012) 805 (https://doi.org/10.1039/C2AN15740C)
G. Saito, W. O. S. Wan Mohd Azman, Y, Nakasugi, T. Akiyama, Adv. Powder Technol. 25(3) (2014) 1038 (https://doi.org/10.1016/j.apt.2014.02.003)
T. D. Malevu, R. O. Ocaya, Int. J. Electrochem. Sci. 9(12) (2014) 8011 (https://doi.org/10.1016/S1452-3981(23)11023-6)
N. Nordin, W. Z. Samad, M. R. Yusup, M. R. Othman, Malaysian J. Anal. Sci. 19 (2015) 236 (https://mjas.analis.com.my/wp-content/uploads/2018/11/Norazzizi_19_1_28.pdf).