Analyzing food supplements to enhance erectile system function for the presence of undeclared sildenafil and tadalafil using HPLC-DAD Short communication
Main Article Content
Abstract
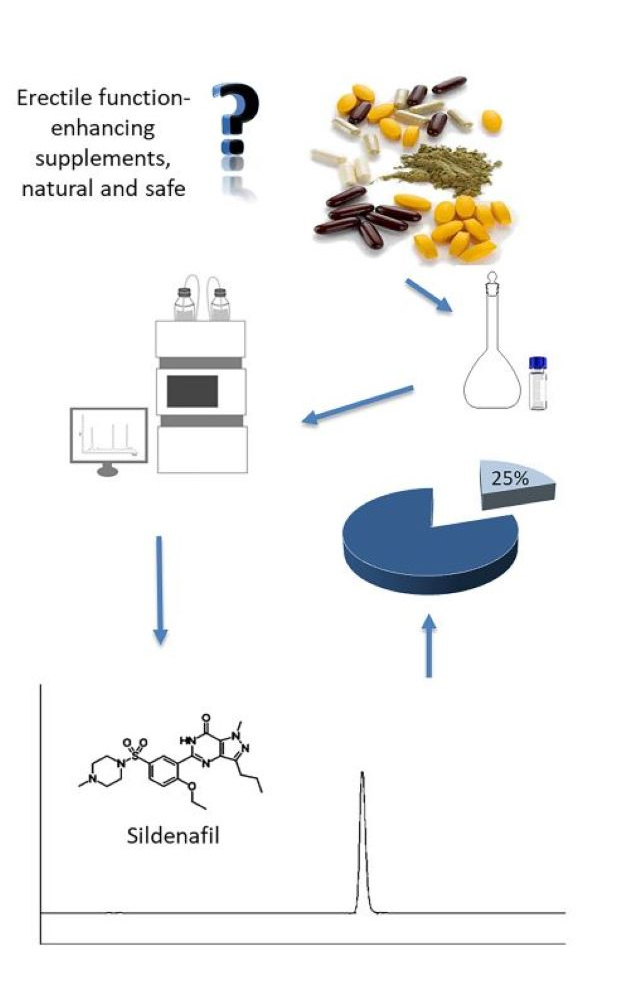
Phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil and tadalafil, are drugs commonly used to improve erectile function. However, some erectile function-enhancing supplements with claims of being entirely natural may be adulterated with these drugs, posing health risks. This study screened supplements for erectile dysfunction from the Republic of North Macedonia using a fast, specific and sensitive HPLC-DAD method (runtime: 15 min). No interference was observed at the retention times of sildenafil and tadalafil, allowing for their unambiguous identification and quantification. Tadalafil was absent, but sildenafil was found in two of eight samples (32.78 and 13.47 mg per dosage). The results demonstrated the applicability of the method to determine whether food supplements marked for the erectile function enhancement are free of sildenafil and tadalafil.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
S. A. Huang, J. D. Lie, Pharm. Ther. 38 (2013) 407 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3776492/)
O. R. C. Gheorghiu, A. M. Ciobanu, C. M. Guțu, C. L. Chițescu, G. V. Costea, D. M. Anghel, A. M. Vlasceanu, D. L. Baconi, Molecules 28 (2023) 4116 (https://doi.org/10.3390/molecules28104116)
L. Muschietti, F. Redko, J. Ulloa, Drug Test. Anal. 12 (2020) 861 (https://doi.org/10.1002/dta.2806)
M. Momtaz, S. Y. Bubli, M. S. Khan, Foods 12 (2023) 199 (https://doi.org/10.3390/foods12010199)
J. T. Dwyer, P.M. Coates, M. J. Smith. Nutrients 10 (2018) 41 (https://doi.org/10.3390/nu10010041)
S. Thakkar, E. Anklam, A. Xu, F. Ulberth, J. Li, B. Li, M. Hugas, N. Sarma, S. Crerar, S. Swift, T. Hakamatsuka, V. Curtui, W. Yan, X. Geng, W. Slikker, W. Tong, Regul. Toxicol. Pharmacol. 114 (2020) 104647 (https://doi.org/10.1016/j.yrtph.2020.104647)
J. Tucker, T. Fischer, L. Upjohn, D. Mazzera, M. Kumar, JAMA Netw. Open 1 (2018) e183337 (https://doi.org/10.1001/jamanetworkopen.2018.3337)
I. A. Ayta, J. B. McKinlay, R. J. Krane, B.J.U.I. 84 (1999) 50 (https://doi.org/10.1046/j.1464-410x.1999.00142.x)
E. Dural, Turk J. Pharm Sci. 17 (2020) 56 (https://doi.org/10.4274/tjps.galenos.2018.91249)
A. K. Podder, J. K. Chakrobarty, A. B. M. Faroque, Asian J. Pharm. Clin. Res.7 (2014) 25 (https://journals.innovareacademics.in/index.php/ajpcr/article/view/987/760)
F. Akuamoa, T. F. H. Bovee, R. van Dam, L. Maro, S. Wesseling, J. Vervoort, I. M. C. M. Rietjens, R. L.A. P. Hoogenboom, Food Addit. Contam., A 39 (2022) 1021 (https://doi.org/10.1080/19440049.2022.2052972)
A. M. Popescu, G. L. Radu, T. Onisei, A. E. Raducacu, C. G. Niculae, Rom. Biotechnol. Lett. 19 (2014) 9493 (https://rombio.unibuc.ro/wp-content/uploads/2022/05/19-4-4.pdf)
D. T. C. Minh, L. A.Thi, N. T. T. Huyen, L.V. Vu, N. T. K. Anh, P. T. Thanh Ha, J. Pharm. Biomed. Anal.174 (2019) 340 (https://doi.org/10.1016/j.jpba.2019.05.043)
C. Mateescu, A. M. Popescu, G. L. Radu, T. Onisei, A. E. Raducanu, Adv. Pharm. Bull. 7 (2017) 251 (https://doi.org/10.15171/apb.2017.030)
C. J. Mwankuna, G. A. Uwamaliya, E. E. Mariki, F. Mabiki, H. M. Malebo, R. Mdegela, B. Styrishave, Anal. Methods 14 (2022) 1060 (http://doi.org/10.1039/d1ay01966j)
R. F. Alotaibi, H. H. Al Tilasi, A. M. Al-Mutairi, H. S. Alharbi, Heliyon 10 (2024) e31467 (http://doi.org/10.1016/j.heliyon.2024.e31467)
J. S. N’cho, I. Fofana, Y. Hadjadj, A. Beroual, Energies 9 (2016) 367 (https://doi.org/10.3390/en9050367)
European Medicines Agency, ICH Q2(R2) Validation of analytical procedures – Scientific guideline, 2022 (https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline)
S. A. K. FIdan, S. Bakirdere, J. AOAC Int. 99 (2016) 923 (https://doi.org/10.5740/jaoacint.15-0320)
M. Tobiszewski, J. Namieśnik, F. Pena-Pereira, Green Chem. 19 (2017)1034 (http://doi.org/10.1039/C6GC03424A)
S. I. Kaya, A. Cetinkaya, S. A. Ozakan, Tr. Env. Anal. Chem. 33 (2022) e00157 (https://doi.org/10.1016/j.teac.2022.e00157)
S. W. A. Sifuma, J. W. Mwangi, P. C. Mutai, D. B. Ongarora, N. M. Njuguna, Int. J. Pharm. Sci. Res. 13 (2022) 2095 (https://doi.org/10.13040/IJPSR.0975-8232.13(5).2095-00)
H. H. Fard, M Akhgari, J. Pharm. Pharmacogn. Res. 6 (2018) 45 (https://doi.org/10.56499/jppres17.265_6.1.45)
C. N. Man, N. M. Nor, R. Lajis, G. L. Harn, J Chromatogr., A 1216 (2009) 8426 (https://doi.org/10.1016/j.chroma.2009.10.016).