Removal of nickel(II) ions during water purification with ferrous sulfate. Part 1. Mechanism and efficiency of the process Scientific paper
Main Article Content
Abstract
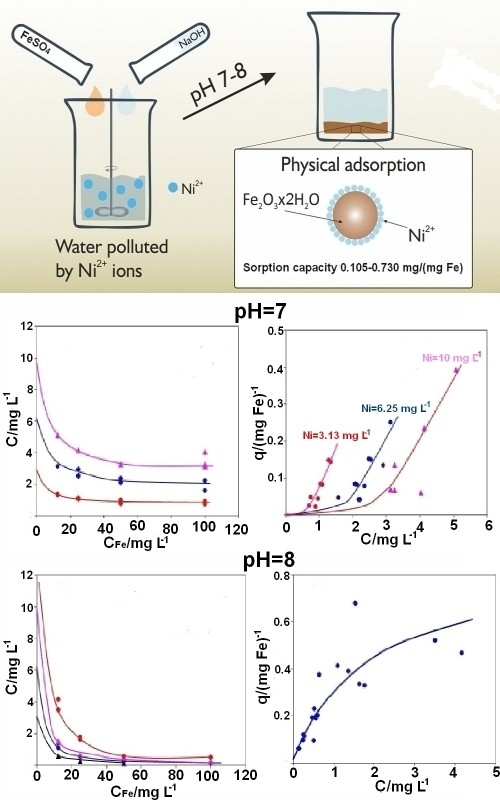
The purification of natural water and wastewater from nickel ions is critically important for both environmental protection and human health due to their high toxicity. This study aimed to investigate the removal of nickel ions from contaminated aqueous solutions using the coagulant FeSO4. The results demonstrate that the removal of nickel ions via an iron(III) hydroxide precipitate, formed during coagulation at pH 7 and pH 8, can be accurately described by classical adsorption isotherms, including the Langmuir, Freundlich and Dubinin–Radushkevich models. The calculated free energy of adsorption, based on the Dubinin–Radushkevich equation, does not exceed 8 kJ mol-1, indicating the physical nature of the adsorption process and ruling out ion-exchange interactions between nickel ions and iron(III) hydroxide. The sorption capacity of the resulting iron(III) hydroxide precipitate for nickel ions at pH 8 is 0.727 mg (mg Fe)-1 of added Fe to the solution. At pH 7, the sorption capacity depends on the initial coagulant concentration and ranges from 0.105 to 0.730 mg (mg Fe)-1. A comparison between the coagulants FeSO4 and the previously studied FeCl3 reveals that FeSO4 is more effective for nickel ion removal when the initial iron ion concentration is below 70 mg L-1. However, at higher initial concentrations of iron, FeCl3 demonstrates greater efficacy.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Russian Foundation for Fundamental Investigations
Grant numbers 20-48-660038 -
Ministry of Science and Higher Education of the Russian Federation
Grant numbers 124020600024-5
References
Monographs on the Evaluation of the Carcinogenic Risks to Humans: Chromium, Nickel and Welding, Vol. 49, IARC, Lyon, 1990
K. Salnikow, A. Zhitkovich, Chem. Res. Tox. 21 (2008) 28 (http://doi.org/10.1021/tx700198a)
M. K. Uddin, Chem. Eng. J. 308 (2017) 438 (http://dx.doi.org/10.1016/j.cej.2016.09.029)
M. Abbasi, E. Safari, M. Baghadi, M. Janmohammadi, J. Water Proc. Eng. 40 (2021) 101961 (http://doi.org/10.1016/j.jwpe.2021.101961)
R. Donat, A. Akdogan, E. Erdem, H. Cetisli, J. Col. Interf. Sci. 286 (2005) 43 (https://doi.org/10.1016/j.jcis.2005.01.045)
Md. A. Islam, Md. R. Awual, M. J. Angove, J. Env. Chem. Eng. 7 (2019) 103305 (http://doi.org/10.1016/j.jece.2019.103305)
S. Kolluru, S. Agarwal, S. Sireesha, I. Sreedhar, S. R. Kale, Proc. Saf. Environ. Prot. 150 (2021) 323 (http://doi.org/10.1016/j.psep.2021.04.025)
V. N. Krasil’nikov, O. D. Linnikov, O. I. Gyrdasova, I. V. Rodina, A. P. Tyutyunnik, I. V. Baklanova, E. V. Polyakov, N. A. Khlebnikov, N. V. Tarakina, Solid State Sci. 108 (2020) 106429 (https://doi.org/10.1016/j.solidstatesciences.2020.106429)
O. D. Linnikov, I. V. Rodina, I. V. Baklanova, A. Yu. Suntsov, Prot. Met. Phys. Chem. Surf. 57(3) (2021) 255 (https://doi.org/10.1134/S2070205121030163)
S. Sivrikaya, S. Albayrak, M. Imamoglu, A. Gundogdu, C. Duran, H. Yildiz, Desal. Water Treat. 50 (2012) 2 (https://doi.org/10.1080/19443994.2012.708234)
M. Vakili, M. Rafatullah, J. Yuan, H. M. Zwain, A. Mojiri, F. Gholami, W. Wang, A. S. Giwa, Y. Yu, G. Cagnetta, G. Yu, Rev. Chem. Eng. 37(6) (2021) 755 (https://doi.org/10.1515/revce-2019-0047)
S. Yang, J. Li, D. Shao, J. Hu, X. Wang, J. Haz. Mat. 166 (2009) 109 (https://doi.org/10.1016/j.jhazmat.2008.11.003)
S. S. Lazarević, I. M. Janković-Častvan, B. M. Jokić, D. T. Janaćković, R. D. Petrović, J. Serb. Chem. Soc. 81(2) (2016) 197 (https://doi.org/10.2298/JSC150525086L)
P. D. Johnson, P. Girinathannair, K. N. Ohlinger, S. Ritchie, L. Teuber, J. Kirby, Water Env. Res. 80(5) (2008) 472 (http://doi.org/10.2175/106143007X221490)
M. A. Inam, R. Khan, K-H. Lee, Y-M. Wie, Int. J. Env. Res. Pub. Health 18 (2021) 9812 (https://doi.org/10.3390/ijerph18189812)
Z. Wu, M. He, X. Guo, R. Zhou, Sep. Pur. Techn. 76 (2010) 184 (https://doi.org/10.1016/j.seppur.2010.10.006)
A. J. Hargreaves, P. Vale, J. Whelan, L. Alibardi, C. Constantino, G. Dotro, E. Cartmell, P. Campo, Clean Techn. Env. Policy 20 (2018) 393 (http://doi.org/10.1007/s10098-017-1481-3)
A. H. Jagaba, S. R. M. Kutty, G. Hayder, L. Baloo, A. A. S. Ghaleb, I. M. Lawal, S. Abubakar, B. N. S. Al-dhawi, N. M. Y. Almahbashi, I. Umaru, Ain Shams Eng. J. 12 (2021) 57 (http://doi.org/10.1016/j.asej.2020.06.016)
O. D. Linnikov, I. V. Rodina, G. S. Zakharova, K. N. Mikhalev, I. V. Baklanova, Yu.V. Kuznetsova, A. Yu. Germov, B. Yu. Goloborodskii, A. P. Tyutyunnik, Z. A. Fattakhova, Water Env. Res. 94(12) (2022) e10827 (https://doi.org/10.1002/wer.10827)
Y. Deng, Water Res. 31(6) (1997) 1347 (https://doi.org/10.1016/s0043-1354(96)00388-0)
M. Kiyama, T. Takada, Bull. Chem. Soc. Japan 45 (1972) 1923
R. R. Kleshcheva, D. A. Zherebtsov, V. Sh. Mirasov, D. G. Kleshchev, Bull. South Ural State Univ. 1 (2012) 17
T. Misawa, K. Yashimoto, S. Shimodaira, Corr. Sci. 14 (1974) 131
Yu. V. Novikov, K. O. Lastochkin, Z. N. Boldina, Methods for studying the quality of water in reservoirs, 2nd ed., Medicine, Moscow, 1990
O. D. Linnikov, I. V. Rodina, Prot. Met. Phys. Chem. Surf. 58 (2022) 1116 (https://doi.org/10.1134/S2070205122060107)
G. Limousin, J-P. Gauder, L. Charlet, S. Szenknect, V. Barthès, M. Krimissa, App. Geochem. 22 (2007) 249 (https://doi.org/10.1016/j.apgeochem.2006.09.010).