Pressure oxidative leaching of copper concentrate Scientific paper
Main Article Content
Abstract
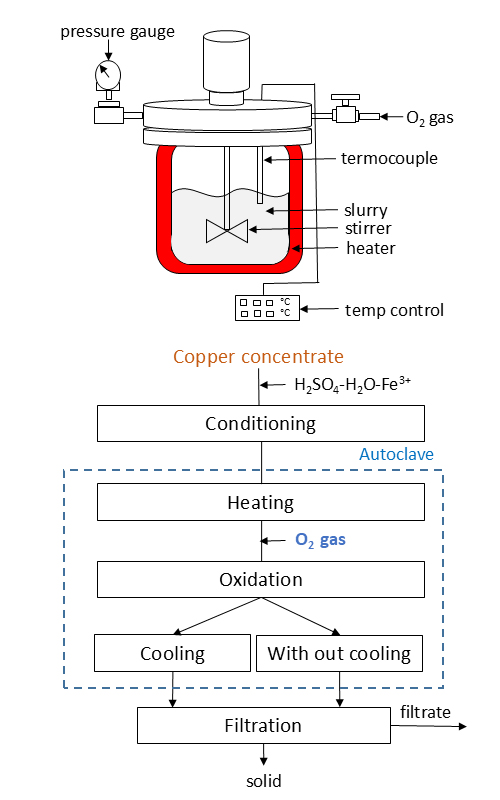
This study investigated the oxidative leaching of copper concentrate using a mixture of ferric ions and sulfuric acid solutions. We examined the effects of various parameters, including temperature, initial sulfuric acid concentration and slurry filtration conditions. At lower temperatures (150 °C), the leaching process resulted in the elemental sulfur and jarosite minerals formed in the solid residue. In contrast, at higher temperatures (190 °C), the solid residue contained jarosite and hematite, the most elemental sulfur-oxidizing to sulfuric acid. Under optimal conditions, a leaching temperature of 190 °C, a concentrate-to-leaching solvent (Fe3+ 5 g L-1 and H2SO4 50 g L-1) ratio of 1:8, an oxygen pressure of 1.0 MPa, and a solid phase particle size of less than 20 μm the dissolution rate of copper reached 98 % after 3 h. When the sulfuric acid concentration increased from 30 to 100 g L-1, the amount of copper increased from 40 to 48 g L-1. Furthermore, rapid filtering of the leaching solution without cooling helped retain most of the iron in the solid phase, resulting in a relatively pure solution. The solid residue was analyzed using X-ray diffraction and scanning electron microscopy.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
Эрдэс баялгийн статистик мэдээ (Mineral Resources and Petroleum Statistics) 2021/08, https://mrpam.gov.mn/public/pages/169/2021.08.stat.report.mon.pdf (accessed: april 2025) (in Mongolian)
N. Tumen-Ulzii, B. Gunchin, J. Serb. Chem. Soc. 88 (2023) 1149 (https://doi.org/10.2298/JSC230114057T)
S. Wang, Jom 57 (2005) 48 (https://doi.org/10.1007/s11837-005-0252-5)
A. A. Baba, K. I. Ayinla, F. A. Adekola, M. K. Ghosh, O. S. Ayanda, R. B. Bale, A. R. Sheik, S. R. Pradhan, Int. J. Min. Eng. Miner. Process. 1 (2012) 1 (https://doi.org/10.5923/j.mining.20120101.01)
J. Cháidez, J. Parga, J. Valenzuela, R. Carrillo, I. Almaguer, Metals (Basel) 9 (2019) 189 (https://doi.org/10.3390/met9020189)
B. Han, B. Altansukh, K. Haga, Y. Takasaki, A. Shibayama, J. Sustain. Metall. 3 (2017) 528 (https://doi.org/10.1007/s40831-017-0135-3)
K. Takatori, H. Kato, A. Yoshimura, Y. Matsuno, Mining, Metall. Explor. 38 (2021) 1477 (https://doi.org/10.1007/s42461-021-00400-3)
M. Sokić, B. Marković, S. Stanković, Ž. Kamberović, N. Štrbac, V. Manojlović, N. Petronijević, Metals (Basel) 9 (2019) 1173 (https://doi.org/10.3390/met9111173)
F. Saloojee, F. K. Crundwell, J. South Afr. Inst. Min. Metall. 116 (2016) 517 (https://doi.org/10.17159/2411-9717/2016/v116n6a5)
S. Heguri, S. Asano, A. Idegami, J. MMIJ 131 (2015) 470 (https://doi.org/10.2473/journalofmmij.131.470)
S. Matuska, K. Ochromowicz, T. Chmielewski, Physicochem. Prob. Miner. Process. 54 (2018) 781–792 (https://bibliotekanauki.pl/articles/110420.pdf)
E. Uzun, M. Zengin, Ý. Atỳlgan, Mater. Tehnol. 50 (2016) 395 (https://doi.org/10.17222/mit.2015.091)
E. M. Córdoba, J. A. Muñoz, M. L. Blázquez, F. González, A. Ballester, Hydrometallurgy 93 (2008) 81 (https://doi.org/10.1016/j.hydromet.2008.04.015)
J. Gega, W. Walkowiak, Physicochem. Probl. Miner. Process. 46 (2011) 155 (https://www.dbc.wroc.pl/Content/10110/46_2011.pdf)
K. J. Nyembwe, E. Fosso-Kankeu, F. Waanders, M. Mkandawire, Trans. Nonferrous Met. Soc. China (Engl. Ed.) 31 (2021) 2139 (https://doi.org/10.1016/S1003-6326(21)65644-3)
S. J. Petrović, G. D. Bogdanović, M. M. Antonijević, M. Vukčević, R. Kovačević, Metals (Basel) 13 (2023) 1818 (https://doi.org/10.3390/met13111818)
S. J. Petrović, G. D. Bogdanović, M. M. Antonijević, Trans. Nonferrous Met. Soc. China (Engl. Ed). 28 (2018) 1444 (https://doi.org/10.1016/S1003-6326(18)64788-0)
H. B. Zhao, M. H. Hu, Y. N. Li, S. Zhu, W. Q. Qin, G. Z. Qiu, J. Wang, Trans. Nonferrous Met. Soc. China (English Ed.) 25 (2015) 303 (https://doi.org/10.1016/S1003-6326(15)63605-6)
Y. Xing, C. Wei, Z. Deng, X. Li, M. Li, Sci. Rep. 14 (2024) 24490 (https://doi.org/10.1038/s41598-024-75857-5)
C. Li, Z. Deng, C. Wei, G. Fan, X. Li, M. Li, Y. Wang, Hydrometallurgy 178 (2018) 294 (https://doi.org/10.1016/j.hydromet.2018.05.012).