Synthesis and biological evaluation of some drug-like scaffolds of benzo- and pyrido-fused medium-sized N-heterocycles obtained via intramolecular Friedel–Crafts acylation reactions Scientific paper
Main Article Content
Abstract
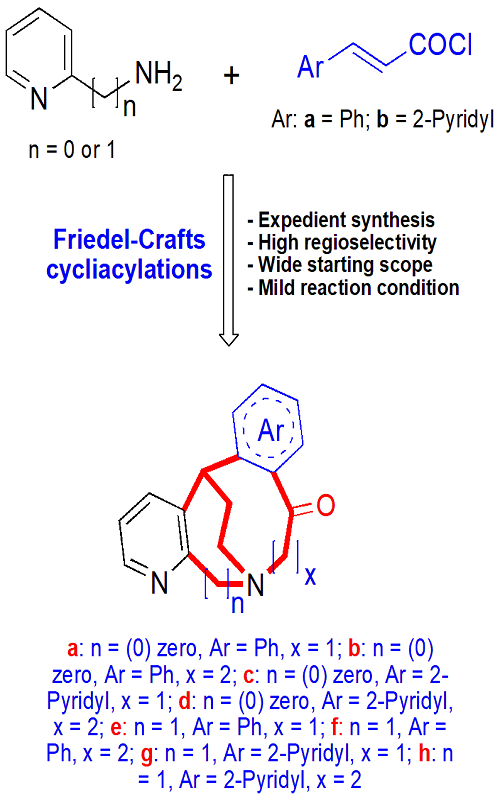
An unprecedented, concise and environmentally-friendly protocol for the synthesis of benzo-and pyrido-annulated azocinones, azoninones and azecinones 8a–h via Friedel–Crafts reactions is described. These simple and efficient procedures involve cycliacylations of heterocyclic esters 7a–h in the presence of catalytic amount of AlCl3/CH3NO2 or TfOH or PPA catalysts as the key step. Starting amides 3a–d were readily obtained by coupling reactions of acryloyl chlorides 2a and b with pyridin-2-amines 1a and b. Developed strategy offers some high selectivity reactions, mild reaction conditions and easy access to complex medium-sized N-heterocycles in moderate to good yields. All tetracyclic fused compounds have been screened for antimicrobial activity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Buckingham, K. H. Baggaley, A. D. Roberts, L. F. Szabó,Dictionary of alkaloids, 2nd ed., CRC Press, Boca Raton, FL, 2010 (ISBN 9780429145377)
D. Robaa, C. Enzensperger, S. E. AbulAzm, M. M. Hefnawy, H. I. E. Subbagh, T. A. Wani, J. Lehmann, J. Med. Chem. 54 (2011) 7422 (https://doi.org/10.1021/jm200676f(
M. J. Moerke, L. R. McMahon, J. L. Wilkerson, Pharm. Rev. 72 (2020) 527 (https://doi.org/10.1124/pr.119.018028)
N. Boden, R. Bissell, J. Clements, B. Movaghar, Liq. Cryst. Today 6 (1996) 1 (https://doi.org/10.1080/13583149608047629)
A. L. Ahmed, Y. Briseno, S. A. Xia, J. Jenekhe, J. Am. Chem. Soc. 130 (2008) 1118 (https://doi.org/10.1021/ja077444g)
C. E. Harding, J. G. R. Standford, J. Org. Chem. 54 (1989) 3054 (https://doi.org/10.1021/jo00274a018)
L. A. Paquette, R. E. Hartung, J. E. Hofferberth, I. Vilotijevic, J. Yang, J. Org. Chem. 69 (2004) 2454 (https://doi.org/10.1021/jo0358675)
N. Krause, C. Winter, Chem. Rev. 111 (2011) 1994 (https://doi.org/10.1021/cr1004088)
R. H. Grubbs, Handbook of Metathesis, Vol. 1, 1st ed., Wiley-VCH, Verlag GmbH, Weinheim, 2003, p. 204
A. T. Soldatenkov, S. V. Volkov, S. A. Soldatova, Chem. Heterocycl. Comp. 43 (2007) 508 (https://doi.org/10.1007/s10593-007-0076-z)
J. Marco-Contelles, E. De Opazo, J. Org. Chem. 67 (2002) 3705 (https://doi.org/10.1021/jo0111107)
H. Ishibashi, T. Sato, M. Ikeda, Synthesis 6 (2002) 695 (https://doi.org/10.1055/s-2002-25759)
M. A. Sierra, I. Fernández, F. P. Cossío, Chem.Commun. (2008) 4671 (https://doi.org/10.1039/B807806H)
D. L. Boger, S. M. Weinreb, Hetero Diels-Alder Methodologyin Organic Synthesis, Academic Press, New York, 1987
L. F. Tietze, G. Brasche, K. M. Gericke, Domino Reactions in Organic synthesis, Wiley‐VCH Verlag GmbH & Co. KgaA, Weinheim, 2006 (ISBN:9783527290604)
J. Limanto, M. L. Snapper, J. Am. Chem. Soc. 122 (2000) 8071 (https://doi.org/10.1021/ja001946b)
T. Hudicky, in Alkaloids: Chemical and Biological Perspectives, S. W. Pelletier, Ed., Wiley-Interscience, New York, Vol. 5, Ch. 1, 1987, pp. 25–37
R. E. Gawley, Org. React. 35 (1987) 1 (https://doi.org/10.1002/0471264180.or035.01)
D. H. R. Barton, R. James, G. W. Kirby, D. W. Turner, D. A. Widdowson, J. Chem. Soc., C (1968) 1529 (https://doi.org/10.1039/J39680001529)
I. W. Elliott, M. J. Sloan, E. Tate, Tetrahedron 52 (1996) 8063 (https://doi.org/10.1016/0040-4020(96)00390-0)
M. Qadir, J. Cobb, P. W. Sheldrake, N. Whittall, A. J. P. White, K. K. Hii, P. N. Horton, M. B. Hursthouse, J. Org. Chem. 70 (2005) 1552 (https://doi.org/10.1021/jo048117j)
L. J. Dolby, D. L. Booth, J. Amer. Chem. Soc. 88 (1966) 1049 (https://doi.org/10.1021/ja00957a035)
N. J. Leonard, T. Sato, J. Org. Chem. 34 (1969) 1066 (https://doi.org/10.1021/jo01256a064)
S. Gerhard, H. Manfred, F. Edgar, B. Heinrich, G. Volker, W. Bernd, S. Wolfgang, European Patent, 0 035 360 B1, 1952
J. B. Bremner, D. F. Perkins, Tetrahedron 61 (2005) 2659 (https://doi.org/10.1016/j.tet.2005.01.061)
G. Kim, M. Y. Chu-Moyer, S. J. Danishefsky, J. Am. Chem. Soc. 112 (1990) 2003 (https://doi.org/10.1021/ja00161a059)
R. Frutos-Pedreño, P. González-Herrero, J. Vicente, P. G. Jones, Organometallics 32 (2013) 1892−1904 (https://doi.org/10.1021/om4000192)
H. A. K. Abd El-Aal, A. A. Khalaf, Arkivoc 8 (2024) 202412254 (https://doi.org/10.24820/ark.5550190.p012.254)
H. A. K. Abd El-Aal, Chem. Heterocycl. Comp. 56 (2020) 1353 (https://doi.org/10.1007/s10593-020-02822-1)
H. A. K. Abd El-Aal, Aus. J. Chem. 76 (2023) 760 (https://doi.org/10.1071/CH23030)
R. M. Roberts, A. A. Khalaf, Friedel-Crafts Chemistry: A Century of Discovery, Marcel Dekker, New York, 1984
G. Pelletier, A. B. Charrette, Org. Lett. 15 (2013) 2290 (https://doi.org/10.1021/ol400870b)
L. Panizzon, Helv. Chim. Acta. 24 (1941) 27 (https://doi.org/10.1002/hlca.19410240205)
L. T. Smith, W. W. Prichard, J. Am. Chem. Soc. 62 (1940) 778 (https://doi.org/10.1021/ja01861a024)
K. H. Yong, J. A. Lotoski, J. M. Chong, J. Org. Chem. 66 (2001) 8248 (https://doi.org/10.1021/jo015940w)
T. Okauchi, I. Masaaki, T. Minami, T. Owa, K. Kitoh, H. Yoshino, Org. Lett. 2 (2000) 1485 (https://doi.org/10.1021/ol005841p)
G. A. Olah, Acc. Chem. Res. 4 (1971) 240 (https://doi.org/10.1021/ar50043a002)
R. D. Shingare, R. Velayudham, J. R. Gawade, D. S. Reddy, Org. Lett. 15 (2013) 4556 (https://doi.org/10.1021/ol402110e)
G. A. Olah, M. Tashiro, S. Kobayashi, J. Am. Chem. Soc. 92 (1970) 6371 (https://doi.org/10.1021/ja00724a063)
R. M. Silverstein, F. X. Webster, D. J. Kiemle, Spectrometric Identification of Organic Compounds, 7th ed., Wiley, Hoboken, NJ, 2005
G. J. Collee, G. A. Fraser, P. B. Marmion, A. Simmons, Practical Medical Microbiology, 14th ed., Churchill Livingstone, Edinburgh, Vol. 11, 1996, p. 163.