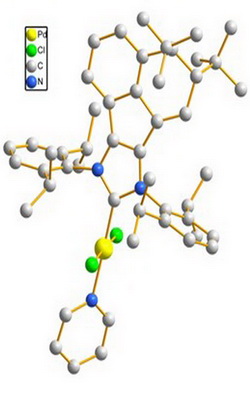
Synthesis and process optimization of Boscalid by catalyst Pd-PEPPSI-IPrDtBu-An
Main Article Content
Abstract
The purpose of this research was to reduce the amount of noble metal palladium catalyst and improve the catalytic performance in the Suzuki-Miyaura cross-coupling reaction, which is the key step in the synthesis of Boscalid. Taking o-bromonitrobenzene and p-chlorophenylboronic acid as raw materials, three kinds of Pd-PEPPSI-IPr catalysts were synthesized and employed in the Suzuki reaction, and then the biaryl product was subjected to reduction and condensation reaction to prepare Boscalid. Under optimal reaction conditions, the result showed that the catalytic system exhibits highest catalytic efficiency under aerobic conditions, giving the 2-(4-chlorophenyl)nitrobenzene over 99 % yield. Moreover, the Pd-PEPPSI-IPrDtBu-An catalyst was minimized to 0.01mol%. The synthesis process was mild, the post-treatment was simple, and the production cost was reduced which makes it suitable for industrial production.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
K. Eicken, K. Goetz, A. Harreus, A. Ammermann, G. Lorenz, A and H. Rang, (BASF AG, Ludwigshafen). European: Patent-EP0545099B (1993)
S. Engel, T. Oberding, (BASF AG, Ludwigshafen). Patent-WO2006/092429A1 (2006)
K. Eicken, M. Rack, F. Wetterich, E. Ammermann, G. Hardt, M. Rack, P. Schäfer, (BASF AG, Ludwigshafen). Patent-WO97/33846A (1997)
C. Torborg, M. Beller, Adv. Synth. Catal. 351 (2009) 3027 (https://doi.org/10.1002/adsc.200900587)
T. N. Glasnov, C. O. Kappe, Adv. Synth. Catal. 17 (2010) 3089 (https://doi.org/10.1002/adsc.201000646)
Y. P. Zhu, S. Sergeyev, P. Franck, R. A. Orru, B. U. W. Maes, Org. Let. 18 (2016) 4602 (https://doi.org/10.1021/acs.orglett.6b02247)
K. S. Manoj, P. M. Siba, G. L. Vinod, B. Ekambaram, Green Chem. 19 (2017) 2111 (https://doi.org/10.1039/C6GC03438A)
R. Dey, B. Sreedhar, B. C. Ranu, Tetrahedron 66 (2010) 2301 (https://doi.org/10.1016/j.tet.2010.02.011)
K. Eicken, M. Rack, F. Wetterich, E. Ammermann, G. Lorenz, S. Strathmann, (BASF AG, Ludwigshafen). German: Patent-DE19735224A1 (1999)
N. Takashi, R. A. Alexander, H. Shenlin, H. L. Bruce, Beilstein. J. Org. Chem. 12 (2016) 1040 (https://doi.org/10.3762/bjoc.12.99)
X. B, Lan, F. M. Chen, B. B. Ma, D. S. Shen, F. S. Liu, Organometallics 35 (2016) 3852 (https://doi.org/10.1021/acs.organomet.6b00723)
T. Tu, W. W. Fang J. Jiang, Chem. Commun. 47 (2011) 12358 (https://doi.org/10.1039/C1CC15503B)
J. OB. Christopher, A. B. K. Eric, V. Cory, H. Niloufar, A. C. Gregory, L. Alan, C. H. Alan, G. O. Michael, Chem. Eur. J. 12 (2006) 4743 (https://doi.org/10.1002/chem.200690054)
F. X. Felpin, E. Fouquet, C. Zakri, Adv. Synth. Catal. 351 (2009) 649 (https://doi.org/10.1002/adsc.200800783)
I. Volovych, M. Neumann, M. Schwarze, RSC Adv. 6 (2016) 58279 (https://doi.org/10.1039/c6ra10484c)
A. Drageset, V. Elumalaia, H. R. Bjørsvik, React. Chem. Eng. 3 (2018) 550 (https://doi.org/10.1039/C8RE00049B)
B. S. Takale, R. R. Thakore, R. Mallarapu, F. Gallou, B. H. Lipshutz, Org. Pro. Res. Dev. 24 (2020) 101 (https://doi.org/10.1021/acs.oprd.9b00455).