Headspace gas chromatography–mass spectrometry method for the determination of total cyanide concentration in water and post-mortem blood samples
Main Article Content
Abstract
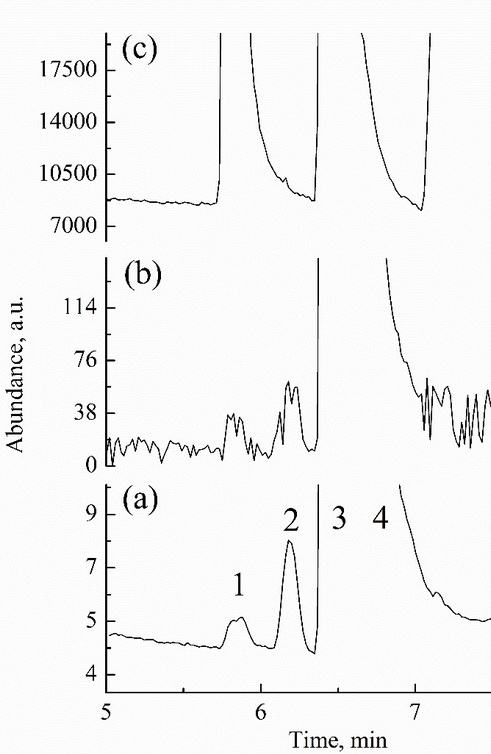
In this study, we aimed to develop a headspace gas chromatography–
–mass spectrometry method for determining the total cyanide concentration in the forensic evidences. Total cyanide content of the samples was calculated based on the hydrogen cyanide gas concentration evaporated from the liquid sample in the headspace vial. Hexacyanoferrate(II) was used for the optimization of headspace oven temperature. We have found that iron–cyanide bonds were completely degraded after 0.2 mL of the sample was treated with 1 mL of 1 M sulfuric acid under the optimized headspace conditions where the temperature and the heating time were 120 °C and 12.5 min, respectively. Satisfactory recovery results for both aqueous and blood samples were obtained. The method was linear in the range 0.05–10 µg mL-1 of cyanide which was a suitable range for toxicological investigations. The proposed method was validated and applied to the post-mortem blood samples, drinking waters, and the other forensic evidences. The proposed method can easily be performed not only in the forensic laboratories, but in the related laboratories where the total cyanide analysis is a critical issue.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
U.S. Environmental Protection Agency, EPA/635/R-08/016F Toxicological Review Of Hydrogen Cyanide And Cyanide Salts, https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0060tr.pdf, (accessed February 17th 2020)
S. Jickells, A. Negrusz, Clarkes Analytical Forensic Toxicology, Pharmaceutical Press, London, 2008, p. 111 (ISBN: 9780853697053)
H. Marquardt, S. Schäfer, R. O. McClellan, F. Welsch, Toxicology, 1st ed., Academic Press-Elsevier, Cambridge, 1999, p. 851 (https://doi.org/10.1016/B978-012473270-4/50094-8)
A. E. Lindsay, A. R. Greenbaum, D. OHare, Anal. Chim. Acta 511 (2004) 185 (https://dx.doi.org/10.1016/j.aca.2004.02.006)
A. Zheng, D. A. Dzomba, R. G. Luthy, B. Sawyer, W. Lazouskas, P. Tata, M. F. Delaney, L. Zilitinkevitch, J. R. Sebroski, R. S. Swartling, S. M. Drop, J. M. Flaherty, Environ. Sci. Technol. 37 (2003) 107 (https://dx.doi.org/10.1021/es0258273)
O. Destanoğlu, G. Gümüş Yılmaz, R. Apak, J. Liq. Chromatogr. Relat. Technol. 38 (2015) 1537 (https://dx.doi.org/10.1080/10826076.2015.1076460)
O. Destanoğlu, G. Gümüş Yılmaz, J. Liq. Chromatogr. Relat. Technol. 39 (2016) 465 (https://dx.doi.org/10.1080/10826076.2016.1192044)
A. E. Lindsay, D. OHare, Anal. Chim. Acta 558 (2006) 158 (https://dx.doi.org/10.1016/j.aca.2005.11.036)
K. E. Murphy, M. M. Schantz, T. A. Butler, B. A. Benner, L. J. Wood, G. C. Turk, Clin. Chem. 52 (2006) 458 (https://dx.doi.org/10.1373/clinchem.2005.061002)
J. Ma, P. K. Dasgupta, Anal. Chim. Acta 673 (2010) 117 (https://dx.doi.org/10.1016/j.aca.2010.05.042)
A. Jain, A. K. K. V. Pillai, N. Sharma, K. K. Verma, Talanta 82 (2010) 758 (https://dx.doi.org/10.1016/j.talanta.2010.05.048)
L. Meng, X. Liu, B. Wang, G. Shen, Z. Wang, M. Guo, J. Chromatogr., B 877 (2009) 3645 (https://dx.doi.org/10.1016/j.jchromb.2009.09.006)
M. Aguilar, A. Farran, V. Martí, Fresenius J. Anal. Chem. 363 (1999) 121 (https://doi.org/10.1007/s002160051153)
A. M. Calafat, S. B. Stanfill, J. Chromatogr., B 772 (2002) 131 (https://dx.doi.org/10.1016/s1570-0232(02)00067-3)
B. Desharnais, G. Huppé, M. Lamarche, P. Mireault, C. D. Skinner, Forensic Sci. Int. 222 (2012) 346 (https://dx.doi.org/10.1016/j.forsciint.2012.06.017)
G. Liu, J. Liu, K. Hara, Y. Wang, Y. Yu, L. Gao, L. Li, J. Chromatogr., B 877 (2009) 3054 (https://dx.doi.org/10.1016/j.jchromb.2009.07.029)
R. K. Bhandari, R. P. Oda, S. L. Youso, I. Petrikovics, V. S. Bebarta, G. A. Rockwood, B. A. Logue, Anal. Bioanal. Chem. 404 (2012) 2287 (https://dx.doi.org/10.1007/s00216-012-6360-5)
S. Kage, T. Nagata, K. Kudo, J. Chromatogr., B 675 (1996) 27 (https://dx.doi.org/10.1016/0378-4347(95)00344-4)
A. A. Cárdenas Riojas, A. Wong, G. A. Planes, M. D. P. T. Sotomayor, A. La Rosa-Toro, A. M. Baena-Moncada, Sensors Actuators, B 287 (2019) 544 (https://dx.doi.org/10.1016/j.snb.2019.02.053)
L. Zhang, H. H. Quan, K. Yang, M. Li, C. P. Chen, J. H. Ahn, J. Hahn, Sensors Actuators, B. 259 (2018) 926 (https://dx.doi.org/10.1016/j.snb.2017.12.143)
A. Yari, R. Sepahvand, Microchim. Acta 174 (2011) 321 (https://dx.doi.org/10.1007/s00604-011-0629-9)
M. L. Koskinen-Soivi, E. Leppämäki, P. Stahlberg, Anal. Bioanal. Chem. 381 (2005) 1625 (https://dx.doi.org/10.1007/s00216-005-3129-0)
C. Zhang, H. Zheng, J. Ouyang, S. Feng, Y. E. C. Taes, Anal. Lett. 38 (2005) 247 (https://dx.doi.org/10.1081/AL-200045143)
A. J. Curtis, C. C. Grayless, R. Fall, Analyst 127 (2002) 1446 (https://dx.doi.org/10.1039/b205378k)
G. Roda, S. Arnoldi, M. D. Cas, V. Ottaviano, E. Casagni, F. Tregambe, G. L. Visconti, F. Farè, R. Froldi, V. Gambaro, J. Anal. Toxicol. 42 (2018) e51 (https://dx.doi.org/10.1093/jat/bky015)
D. Marton, A. Tapparo, V. B. Di Marco, C. Repice, C. Giorio, S. Bogialli, J. Chromatogr., A 1300 (2013) 209 (https://dx.doi.org/10.1016/j.chroma.2013.03.004)
V. Gambaro, S. Arnoldi, E. Casagni, L. DellAcqua, C. Pecoraro, R. Froldi, J. Forensic Sci. 52 (2007) 1401 (https://dx.doi.org/10.1111/j.1556-4029.2007.00570.x)