CO oxidation over alumina monolith impregnated with oxides of copper and manganese Scientific paper
Main Article Content
Abstract
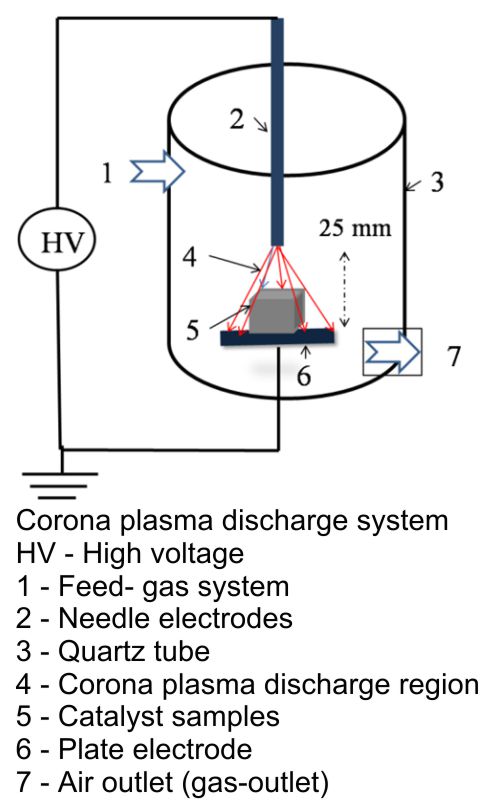
In this work, simple methods for the preparation of highly efficient heterogeneous nanocatalysts for the low-temperature oxidation of CO are described. The main advantages of the reaction are high yields. The catalysts based on oxides of copper and manganese supported on alumina monoliths were prepared by different methods: plasma corona discharge and wet impregnation. Structure and physical properties of catalysts were characterized by FT-
-IR, XRD, TEM, EDX and TG/DTA. The results showed that the use of a plasma corona discharge at atmospheric pressure for the preparation of the catalysts resulted in smaller particle size and uniform dispersion when compared with the catalysts prepared by wet impregnation methods. The catalytic activities of these catalysts were investigated for complete oxidation of carbon monoxide (3000 ppm) to carbon dioxide in the air at atmospheric pressure. On a single oxide catalyst, 10CuO/monolith was better than 10MnO2/monolith under the same experimental conditions. With multi-oxide catalysts, all catalyst samples are more active than a single-oxide catalyst with the same impregnated content. In particular, the catalyst prepared by plasma corona discharge indicates the best oxidation capacity of carbon monoxide.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
H. Kinoshita, H. Türkan, S. Vucinic, S. Naqvi, R.Bedair, R. Rezaee, A. Tsatsakis, Toxicol. Rep. 7 (2020) 169 (https://doi.org/10.1016/j.toxrep.2020.01.005)
R. J. Levy, Neurotoxicol. Teratol. 49 (2015) 31 (https://doi.org/10.1016/j.ntt.2015.03.001)
J. Xu, T. White, P. Li, C. He, J. Yu, W. Yuan. Y.-F. Han, J. Am. Chem. Soc. 132 (2010) 10398 (https://doi.org/10.1021/ja102617r)
A. S. Ivanova, E. M. Slavinskaya, R. V. Gulyaev, V. I. Zaikovskii, О. А. Stonkus, I. G. Danilova. L. M. Plyasova, I. A. Polukhin, A. I. Boronin, Appl. Catal. B: Environ. 97 (2010) 57 (https://doi.org/10.1016/j.apcatb.2010.03.024)
H. Huang, D. Y. C. Leung, D. Ye, J. Mater. Chem. 21 (2011) 9647 (https://doi.org/10.1039/C1JM10413F)
Y. Lang, J. Zhang, Z. Feng, X. Liu, Y. Zhu, T. Zeng, Y. Zhao, R. Chen, B. Shan, Catal. Sci. Technol. 8 (2018) 5490 (https://doi.org/10.1039/C8CY01263F)
D. A. Aguilera, A. Perez, R. Molina, S. Moreno, Appl. Catal., B 104 (2011) 144 (https://doi.org/10.1016/j.apcatb.2011.02.019)
M. R. Morales, B. P. Barbero, L. E. Cadús, Fuel 87 (2008) 1177 (https://doi.org/10.1016/j.fuel.2007.07.015)
P. W. Park, J. S. Ledford, Appl. Catal., B 15 (1998) 221 (https://doi.org/10.1016/S0926-3373(98)80008-8)
K. Y. Koo, U. H. Jung, W. L. Yoon, Int. J. Hydrogen Energy 39 (2014) 5696 (https://doi.org/10.1016/j.ijhydene.2014.01.128)
Z.-H. Li, S.-H. Tian, H.-T. Wan, H.-B. Tian, J. Mol. Catal., A 211 (2004) 149 (https://doi.org/10.1016/j.molcata.2003.10.003)
W. Hua, L. Jin, X. He, J. Liu, H. Hu, Catal. Commun. 11 (2010) 968 (https://doi.org/10.1016/j.catcom.2010.04.007)
M. H. Chen, W. Chu, X. Y. Dai, X. W. Zhang, Catal. Today 89 (2004) 201 (https://doi.org/10.1016/j.cattod.2003.11.027)
S. Dey, G. C. Dhal, D. Mohan, R. Prasad, Bull. Chem. React. Eng. Catal. 12 (2017) 437 (https://doi.org/10.9767/bcrec.12.3.900.437-451)
D. P. Dubal, G. S. Gund, C. D. Lokhande, R. Holze, Mat. Res. Bull. 48 (2013) 923 (https://doi.org/10.1016/j.materresbull.2012.11.081)
M. Aghazadeh, M. Asadi, M. G. Maragheh, M. R. Ganjali, P. Norouzi, F. Faridbod, App. Surf. Sci. 364 (2016) 726 (https://doi.org/10.1016/j.apsusc.2015.12.227).