Trace determination of isoniazid at micro level using kinetic spectrophotometric method Scientific paper
Main Article Content
Abstract
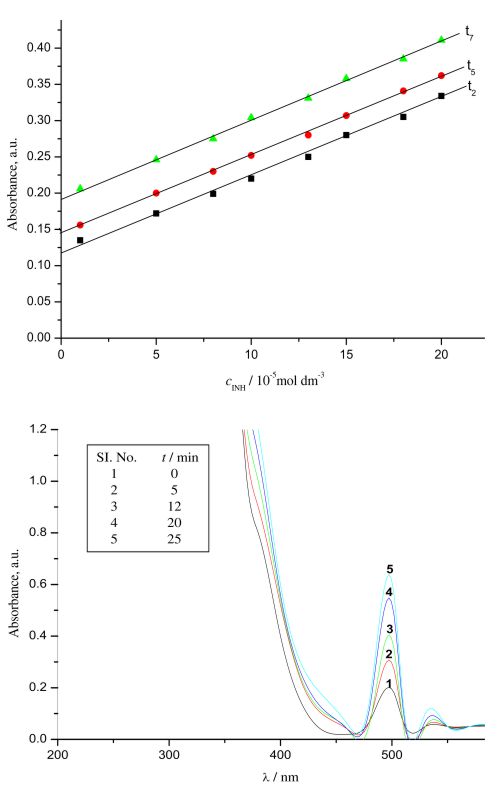
An effective and fairly inexpensive spectrophotometric method for tracing the determination of isoniazid (INH) in pure form, as well as in pharmaceutical formulations, has been developed through the ligand substitution reaction between INH and aquapentacyanoruthenate (II) ion ([Ru(CN)5OH2]3-) in aqueous medium at λmax = 502 nm. The fixed time procedure has been employed under optimum reaction conditions. The calibration equations, relating absorbance measured at 502 nm at fixed times (tn = 2, 5 and 7 min) and cINH in linear range 1.37‑27.43 µg mL-1, were used for the trace determination of INH, which has been reported in the present investigation and are in agreement with official and reported methods. The percentage recovery has been calculated and found to be within the range of 99–101 % in the analysis of different pharmaceutical samples. The results reveal that the use of common recipients as the used additives do not produce any type of interference in the suggested method. The validity of the proposed method was also checked by statistical analysis which agreed with the results obtained using the official method. The present method is very simple, reproducible, sensitive and it can be adopted for trace determination of INH in different samples without using extracting agent.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
Global Tuberculosis Report 2012, World Health Organization, 2012 (https://apps.who.int/iris/handle/10665/75938)
A. Korokovals, J. H. Burckhalter, J. Chem. Educ. 54 (1977) A497. (https://dx.doi.org/10.1021/ed054pA497.2)
H. Meyer, J. Mally, Monatsh. Chem. 33 (1912) 393. (https://dx.doi.org/10.1007/BF01517946)
H. H. Fox, Science 116 (1952) 129 (https://www.jstor.org/stable/1680129)
C. Vilcheze,W. R. Jacobs, Ann. Rev. Microbiol. 61 (2007) 35 (https://dx.doi.org/10.1146/annurev.micro.61.111606.122346)
K. Johnsson, D. S. King, P. G. Schultz, J. Am. Chem. Soc. 117 (1995) 5009. (https://dx.doi.org/10.1021/ja00122a038)
D. A. Rozwarski, G. A. Grant, D. H. R. Barton, W. R. Jabobs, J. C. Sacchettini, Science 279 (1998) 98 (https://dx.doi.org/10.1126/science.279.5347.98)
A. M. El-Bbrashy, L. R. Elhussein, Anal. Lett. 30 (1997) 609 (https://dx.doi.org/10.1080/00032719708001805)
Q.-M. Li, Z.-J. Yang, J. Chin. Chem. Soc. 53 (2006) 383 (https://dx.doi.org/10.1002/jccs.200600049)
J. V. de Assis, M. G. Teixeira, C. G. P. Soares, J. F. Lopes, G. S. L. Carvalho, M. C. S. Lourenço, M. V. de Almeida, W. B. de Almeida, S. A. Fernandes, Eur. J. Pharm. Sci. 47 (2012) 539. (https://dx.doi.org/10.1016/j.ejps.2012.07.015)
J. A.García Bautista, J. V.García Mateo, J. M. Calatayud, Anal. Let. 31 (1988) 1209 (http://dx.doi.org/10.1080/00032719808002857)
Z. Q. Zhang, Z. X. Cao, X. M. He, X. M. Li, Y. F. Li, J. Anal. Sci. 12 (1996) 52 (http://en.cnki.com.cn/Article_en/CJFDTotal-FXKX199601015.htm)
J. Xi, B. Shi, X. Ai, Z. He, J. Pharm. Biomed. Anal. 36 (2004) 237 (https://dx.doi.org/10.1016/j.jpba.2004.05.021)
M. Acedo-Valenzuela, A. Espinosa-Mansilla, A. M. D. Pena, F. Canada-Canada, Anal. Bioanal. Chem. 374 (2002) 432. (http://dx.doi.org/10.1007/s00216-002-1494-5)
B. Haghighi, S. Bozorgzadeh, Microchem. J. 95 (2010) 192. (https://dx.doi.org/10.1016/j.microc.2009.11.012)
M. A. Karimi, M. Mazloum-Ardakani, M. H. Mashhadizadeh, F. Banifatemeh, Croat. Chem. Acta 82 (2009) 729 (https://hrcak.srce.hr/45534)
R. M. Kulkarni, D. C. Bilehal, S. T. Nandibewoor, Anal. Sci. 20 (2004)743 (https://dx.doi.org/10.2116/analsci.20.743)
M. R. Majidi, A. Jouyban, K. Asadpour-Zeynali, J. Electroanal. Chem. 89 (2006) 32 (https://dx.doi.org/10.1016/j.jelechem.2006.01.016)
J. S. Singh. Res. J. Pharm. Technol. 13 (2020) 4061 (https://doi.org/10.5958/0974-360X.2020.00718.0)
M. Y. Khuhawar, L. A. Zardari, J. Food Drug Anal. 14 (2006) 323 (http://iarscs.usindh.edu.pk/myk/papers/2006/1442p323328.pdf)
D. Hebel, S. Guermouche, M. H. Guermuche, JPC-J. Planar. Chromat. 10 (1997) 453 (http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=2127215)
S. Guermouche, M. H. Guermouche, J. Chromatogr. Sci. 42 (2004) 250 (https://dx.doi.org/10.1093/chromsci/42.5.250)
M. Acedo-Valenzuela, A. Espinosa-Mansilla, A. M. D. Pena, F. Canada-Canada, Anal. Bioanal. Chem. 374 (2002) 432 (https://dx.doi.org/10.1007/s00216-002-1494-5)
I. L. Tsai, H. Y. Liu, P. H. Kuo, J. Y. Wang, L. J. Shen, C. H. Kuo, Anal. Bioanal. Chem. 401 (2011) 2205 (https://dx.doi.org/10.1007/s00216-011-5285-8)
M. Y. Khuhawar, F. M. A. Rind, J. Chromatogr., B 766 (2002) 357 (https://dx.doi.org/10.1016/S0378-4347(01)00510-2)
M. C. Gennaro, R. Calvino, C. Abrigo, J. Chromatogr., B 754 (2001) 477 (https://dx.doi.org/10.1016/S0378-4347(01)00037-8)
A. Safavi, M. Bagheri, Spectrochim. Acta, A 70 (2008)735 (https://dx.doi.org/10.1016/j.saa.2007.09.001)
G. F. Dos. S. Fernandes, H. Regina. Nunes Salgado, J. L. D.Santos, Crit. Rev. Anal. Chem. 47 (2017) 298 (https://doi.org/10.1080/10408347.2017.1281098)
R. M. Naik, A. Agarwal, S. Prasad, Spectrochim. Acta, A 74 (2009) 887 (https://dx.doi.org/10.1016/j.saa.2009.08.029)
R. M. Naik, A. Agarwal, S. Prasad, A. K. Verma, Microchem. J. 93 (2009) 43 (https://dx.doi.org/10.1016/j.microc.2009.04.006)
R. M. Naik, J. Sarkar, S. Prasad, Microchem. J. 88 (2008) 45 (https://dx.doi.org/10.1016/j.microc.2007.09.003)
S. Prasad, R. M. Naik, A. Srivastava, Spectrochim. Acta, A 70 (2008) 958 (https://dx.doi.org/10.1016/j.saa.2007.10.011)
R. M. Naik, B. Kumar, A. Asthana, Spectrochim. Acta, A 75 (2010) 1152 (https://dx.doi.org/10.1016/j.saa.2009.12.078)
A. Agarwal, S. Prasad, R. M. Naik, Microchem. J. 128 (2016) 181 (https://dx.doi.org/10.1016/j.microc.2016.04.005)
R. M. Naik, S. Prasad, B. Kumar, S. B. S. Yadav, A. Asthana, M. Yoshida, Microchem. J. 111 (2013) 108 (https://dx.doi.org/10.1016/j.microc.2013.02.011)
V. Chand, S. Prasad, J .Hazard. Mater. 165 (2009) 780 (https://dx.doi.org/10.1016/j.jhazmat.2008.10.076)
United States Pharmacopoeia XXIV, U.S.P. Convention, Rockville, MD20852, USA, 2000 (https://www.pharmaceuticalonline.com/doc/united-states-pharmacopoeia-xxiv-national-for-0001
A. I. Vogel, J. Bassett, Vogels text book of quantitative inorganic analysis, 4th ed., Longman, New York, 1978 (ISBN-13: 978-0582463219)
R.C. Weast, CRC Handbook of Chemistry and Physics, 60th ed., CRC Press, Boca Raton, FL, 1979 (ISBN-13, https://www.biblio.com/9780849304606)
C. R. Johnson, R. E. Shepherd, Inorg. Chem. 22 (1983) 2439 (https://dx.doi.org/10.1021/ic00159a020)
K. W. Hicks, G. Chappelle, Inorg. Chem. 19 (1980) 1623 (https://dx.doi.org/10.1021/ic50208a038).