Investigation of selective leaching conditions of ZnO, ZnFe2O4 and Fe2O3 in electric arc furnace dust in HNO3 Scientific paper
Main Article Content
Abstract
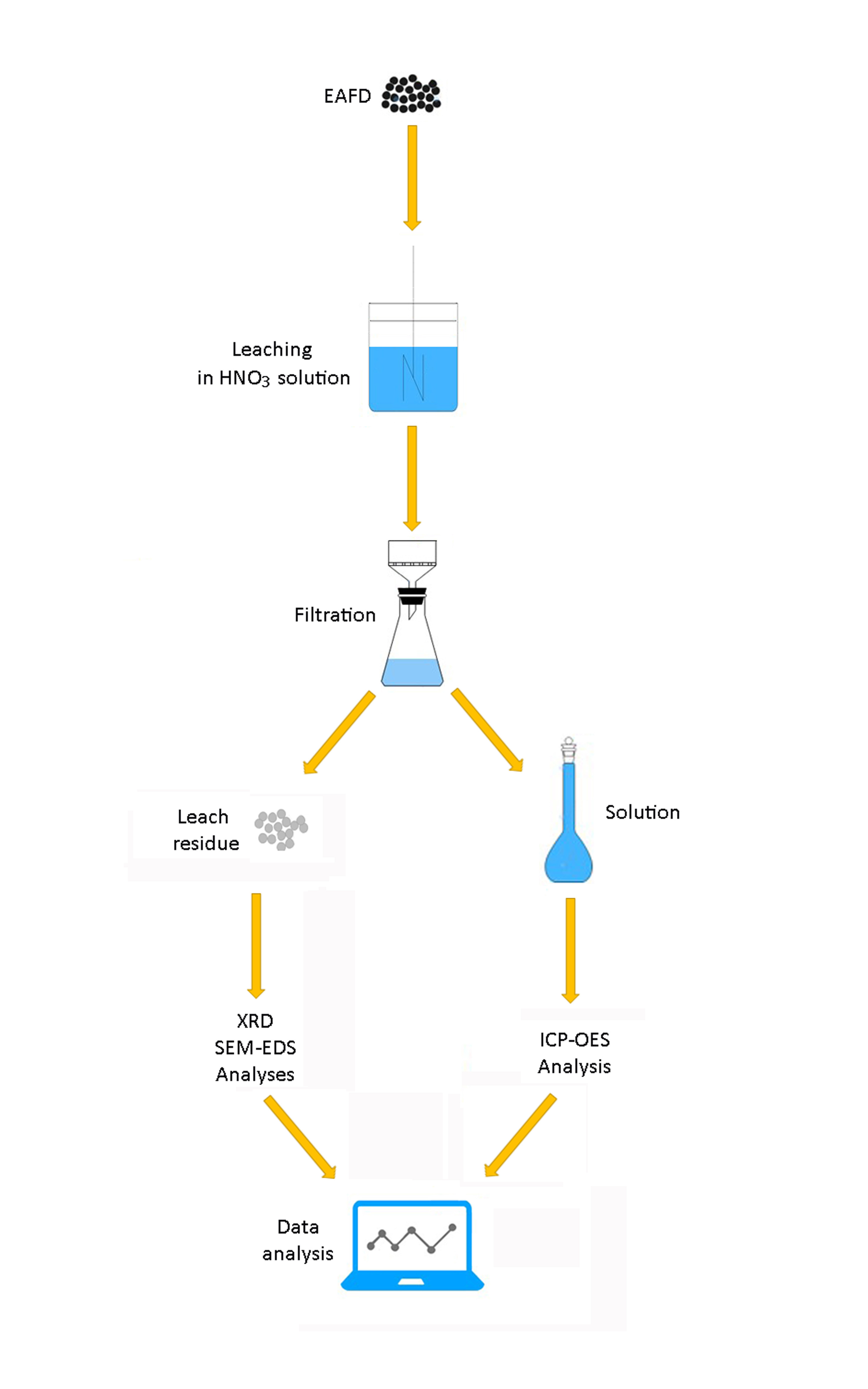
Electric arc furnace dust (EAFD) includes mainly Zn, Fe, Pb, Ca and Mn-bearing compounds. Thus, EAFD is classified as a hazardous waste. The dissolution behavior of Zn- and Fe-bearing compounds in EAFD in nitric acid solutions was investigated in this work. The composition of Zn- and Fe-bearing compounds in the EAFD was determined as 28.58, 37.96 and 11.33 % for ZnO, ZnFe2O4 and Fe2O3, respectively. The effect of stirring speed, temperature and HNO3 concentration on the dissolution rate of ZnO, ZnFe2O4 and Fe2O3 were investigated and optimum leaching conditions determined. While ZnO was dissolved rapidly, the dissolution rate of ZnFe2O4 increased with increasing temperature and HNO3 concentration. Fe2O3 was not soluble in 0.5 M HNO3 solution at 40 °C, whereas it was dissolved completely in 4 M HNO3 solution at 80 °C.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
P. Oustadakis, P. E. Tsakiridis, A. Katsiapi, S. Agatzini–Leonardou , J. Hazard. Mater. 179 (2010) 1 (https://doi.org/10.1016/j.jhazmat.2010.01.059)
T. Havlik, M. Turzakova, S. Stopic, B. Friedrich, Hydrometallurgy 77 (2005) 41 (https://doi.org/10.1016/j.hydromet.2004.10.008)
The Union of Chambers and Commodity Exchanges of Turkey, Turkish Ferrous and Nonferrous Metals Council Report, 2016, https://www.tobb.org.tr/Documents/yayinlar/EkonomikRapor/Eng/2017%20Economic%20Report.pdf (accessed 23/10/2018)
T. Havlík, B. De Souza, A. M. Bernardes, I.A.H. Schneider, A. Miškufová, J. Hazard. Mater. 135 (2006) 311 (https://doi.org/10.1016/j.jhazmat.2005.11.067)
J. R. Donald, C. A. Pickles, Can. Metall. Q. 35 (1996) 255 (https://doi.org/10.1016/0008-4433(96)00009-2)
N. Štrbac, I. Mihajlović, V. Andrić, Ž. Živković, A. Rosić, Can. Metall. Q. 50 (2011) 28 (https://doi.org/10.1179/000844311X552287)
M. H. Morcali, O. Yucel, A. Aydin, B. Derin, J. Min. Metal. 48 (2012) 173 (https://doi.org/10.2298/JMMB111219031M)
X. Lina, Z. Penga, J. Yana, Z. Li, J.Y. Hwang, Y. Zhang, G. Li, J. Clean. Prod. 149 (2017) 1079 (https://doi.org/10.1016/j.jclepro.2017.02.128)
V. N. R. Sarma, K. Deo, A. K. Biswas, Hydrometallurgy 2 (1976) 171 (https://doi.org/10.1016/0304-386X(76)90026-8)
M. Cruells, A. Roca, C. Núnẽz, Hydrometallurgy 32 (1992) 213 (https://doi.org/10.1016/0304-386X(92)90119-K)
C. Caravaca, A. Cobo, F. J. Alguacil, Resour. Conserv. Recycl. 10 (1994) 35 (https://doi.org/10.1016/0921-3449(94)90036-1)
D. K. Xia, C. A. Picklesi, Miner. Eng. 13 (2000) 79 (https://doi.org/10.1016/S0892-6875(99)00151-X)
Z. Youcai, R. Stanforth, Miner. Eng. 13 (2000) 1417 (https://doi.org/10.1016/S0892-6875(00)00123-0)
N. Leclerc, E. Meux, J. M. Lecuire, Hydrometallurgy 70 (2003) 175 (https://doi.org/10.1016/S0304-386X(03)00079-3)
T. Havlik, B. Friedrich, S. Stopic, World Metal. – ERZMETALL 57 (2004) 83 (http://www.metallurgie.rwth-aachen.de/new/images/pages/publikationen/havlik_erzmetall_57_id_9401.pdf)
S. Kelebek, S. Yoruk, B. Davis, Miner. Eng. 17 (2004) 285 (https://doi.org/10.1016/j.mineng.2003.10.030)
G. Orhan, Hydrometallurgy 78 (2005) 236 (https://doi.org/10.1016/j.hydromet.2005.03.002)
A. J. B. Dutra, P. R. P. Paiva, L. M. Tavares, Miner. Eng. 19 (2006) 478 (https://doi.org/10.1016/j.mineng.2005.08.013)
R. A. Shawabkeh, Hydrometallurgy 104 (2010) 61 (https://doi.org/10.1016/j.hydromet.2010.04.014)
F. Kukurugya, T. Vindt, T. Havlík, Hydrometallurgy 154 (2015) 20 (https://doi.org/10.1016/j.hydromet.2015.03.008)
N. Peng, B. Peng, H. Liu, D. H. Lin, K. Xue, Can. Metall. Q. 56 (2017) 301 (https://doi.org/10.1080/00084433.2017.1343174)
R. L. Nyirenda, Miner. Eng. 4 (1991) 1003 (https://doi.org/10.1016/0892-6875(91)90080-F).
B. Boyanov, A. Peltekov, K. Ivanov, Int. J. Chem. Mol. Eng. 9 (2015) 765 (https://publications.waset.org/10002705/pdf)
Š. Langová, J. Riplová, S. Vallová, Hydrometallurgy 87 (2007) 157 (https://doi.org/10.1016/j.hydromet.2007.03.002)
Z. Sedláková, D. Orac, T. Havlik, Acta Metall. Slovaca 12 (2006) 338 (https://www.censo.fmmr.tuke.sk/content/clanky/200606.pdf)
T. Havlik, F. Kukurugya, D. Orac, L. Parilak, World Metal. – ERZMETALL 65 (2012) 48 (https://www.researchgate.net/publication/279701830_Acidic_leaching_of_EAF_steelmaking_dust)
M. C. Da Silva, A. M. Bernardes, C. P. Bergmann, J. A. S. Tenório, D. C. R. Espinosa, Ironmak. Steelmak. 35 (2008) 315 (https://doi.org/10.1179/030192307X232936).