Microwave-assisted synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives and their biological evaluation
Main Article Content
Abstract
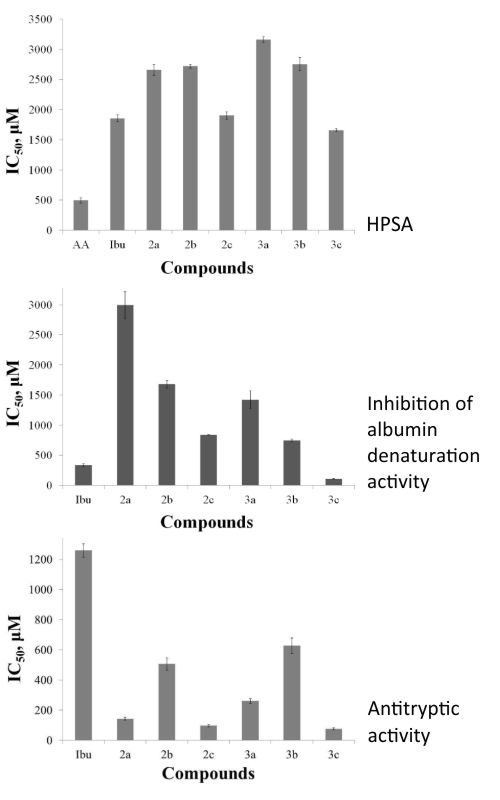
Herein we report an alternative eco-friendly method for the synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives. All obtained compounds were screened for their in vitro inhibition of albumin denaturation, antioxidant, antitryptic and antibacterial activity, and have shown significant results. The lipophilicity was established using both reversed-phase thin layer chromatography and in silico calculations.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
R. N. Shelke, D. N. Pansare, A. P. Sarkate, I. K. Narula, D. K. Lokwani, S. V Tiwari, R. Azad, S. R. Thopate, Bioorg. Med. Chem. Lett. 30 (2020) 127246 (https://doi.org/10.1016/j.bmcl.2020.127246)
R. Pingaew, P. Mandi, C. Nantasenamat, S. Prachayasittikul, S. Ruchirawat, V. Prachayasittikul, Eur. J. Med. Chem. 81 (2014) 192 (https://doi.org/10.1016/j.ejmech.2014.05.019)
G. Blaskó, P. Kerekes, S. Makleit, Reissert Synthesis of Isoquinoline and Indole Alkaloids in The Alkaloids: Chemistry and Pharmacology, A. Brossi (Ed.), Elsevier, Academic Press, New York, 1987, p. 1 (https://doi.org/10.1016/S0099-9598(08)60256-4)
M. Shamma, in The Isoquinoline Alkaloids. Chemistry and Pharmacology, Academic Press, New York, 1972, p. 595 (https://doi.org/10.1016/B978-0-12-638250-1.50040-X)
G. Jones, in Comprehensive Heterocyclic Chemistry II, A. R. Katritzky, C. V. Rees, E. F. V. Scriven (Eds.), Pergamon Press, Elsevier Sci. Ltd., New York, 1997 (https://doi.org/10.1021/jm9706123)
R. Kreher, S. Andreae, S. von Angerer, P. Czerney, H. Hartmann, M. A. Kessler, E. Reimann, W.-D. Rudorf, I. Stahl, O. S. Wolfbeis, in Methoden der Organischen Chemie (Houben-Weyl), E7a, 4th ed., Georg Thieme Verlag, Stuttgart, 1991, p. 571 (https://www.thieme-connect.de/products/ebooks/book/10.1055/b-003-115784)
K. W. Bentley, Nat. Prod. Rep. 9 (1992) 365 (http://dx.doi.org/10.1039/NP9920900365)
K. W. Bentley, Nat. Prod. Rep. 17 (2000) 247 (http://dx.doi.org/10.1039/A900251K)
A. K. Pathak, C. Ameta, R. Ameta, P. B. Punjabi, J. Heterocycl. Chem. 53 (2016) 1697 (https://doi.org/10.1002/jhet.2515)
B. T. Pérez-Martínez, M. A. Aboudzadeh, U. S. Schubert, J. R. Leiza, R. Tomovska, Chem. Eng. J. 399 (2020) 125761 (https://doi.org/10.1016/j.cej.2020.125761)
H. M. Nguyen, J. Sunarso, C. Li, G. H. Pham, C. Phan, S. Liu, Appl. Catal., A 599 (2020) 117620 (https://doi.org/10.1016/j.apcata.2020.117620)
E. Awuah, A. Capretta, J. Org. Chem. 75 (2010) 5627 (https://doi.org/10.1021/jo100980p)
I. D. Lick, L. Gavernet, L. E. Bruno-Blanch, E. N. Ponzi, Thermochim. Acta 501 (2010) 30 (https://doi.org/10.1016/j.tca.2009.12.019)
R. J. Ruch, S.-J. Cheng, J. E. Klaunig, Carcinogenesis 10 (1989) 1003 (https://doi.org/10.1093/carcin/10.6.1003)
S. S. Sakat, A. R. Juvekar, M. N. Gambhire, Int. J. Pharm. Pharm. Sci. 2 (2010) 146 (https://innovareacademics.in/journal/ijpps/Vol2Issue1/322.pdf)
O. O. Oyedapo, A. J. Famurewa, Int. J. Pharmacogn. 33 (1995) 65 (https://doi.org/10.3109/13880209509088150)
I. Wiegand, K. Hilpert, R. E. W. Hancock, Nat. Protoc. 3 (2008) 163 (https://doi.org/10.1038/nprot.2007.521)
E. Pontiki, D. Hadjipavlou-Litina, Bioorg. Med. Chem. 15 (2007) 5819 (https://doi.org/10.1016/j.bmc.2007.06.001)
A. Sadym, A. Lagunin, D. Filimonov, V. Poroikov, SAR QSAR Environ. Res. 14 (2003) 339 (https://doi.org/10.1080/10629360310001623935)
D. A. Filimonov, A. A. Lagunin, T. A. Gloriozova, A. V. Rudik, D. S. Druzhilovskii, P. V. Pogodin, V. V. Poroikov, Chem. Heterocycl. Compd. 50 (2014) 444 (http://link.springer.com/10.1007/s10593-014-1496-1)
S. Manolov, S. Nikolova, I. Ivanov, Molecules 18 (2013) 1869 (https://www.mdpi.com/1420-3049/18/2/1869)
C. Schotten, Berichte Der Dtsch. Chem. Gesellschaft 17 (1884) 2544 (https://doi.org/10.1002/cber.188401702178)
E. Baumann, Berichte Der Dtsch. Chem. Gesellschaft 19 (1886) 3218 (https://doi.org/10.1002/cber.188601902348)
A. A. H. Kadhum, A. A. Al-Amiery, A. Y. Musa, A. B. Mohamad, Int. J. Mol. Sci. 12 (2011) 5747 (https://doi.org/10.3390/ijms12095747)
J. H. Naama, G. H. Alwan, H. R. Obayes, A. A. Al-Amiery, A. A. Al-Temimi, A. A. H. Kadhum, A. B. Mohamad, Res. Chem. Intermed. 39 (2013) 4047 (http://link.springer.com/10.1007/s11164-012-0921-2)
M. Ebrahimzadeh, S. Nabavi, S. Nabavi, F. Bahramian, A. Bekhradnia, Pak. J. Pharm. Sci. 23 (2010) 29 (https://pubmed.ncbi.nlm.nih.gov/20067863)
J. R. Vane, R. M. Botting, Inflamm. Res. 44 (1995) 1 (http://link.springer.com/10.1007/BF01630479)
V. Jayashree, S. Bagyalakshmi, K. Manjula Devi, D. Richard Daniel, Asian J. Pharm. Clin. Res. 9 (2016) 108 (https://doi.org/10.22159/ajpcr.2016.v9s2.12623)
C. Hansch, A. Leo, D. Hoekman, Exploring QSAR: hydrophobic, electronic, and steric constants, American Chemical Society, Washington, DC, 1995, ISBN-13: 978-0841229914 (https://www.amazon.com/Exploring-QSAR-Hydrophobic-Electronic-Professional/dp/0841229910)
K. S. Joseph, J. Anguizola, D. S. Hage, J. Pharm. Biomed. Anal. 54 (2011) 426 (https://doi.org/10.1016/j.jpba.2010.09.003)
G. R. Behbehani, M. Hossaini Sadr, H. Nabipur, L. Barzegar, J. Chem. 2013 (2013) 1 (https://doi.org/10.1155/2013/120480).